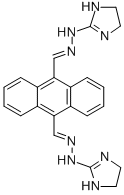
The biological activity and cytotoxicity of Bisantrene
Description
Bisantrene is an organic compound with the chemical formula (9,10-anthracenedicarboxaldehyde bis [(4,5-dihydro-1H-imidazol-2yl)hydrazone] dihydrochloride). This compound is a known intercalating agent endowed with topoisomerase-II poisoning activity[1-2].
Biological activity
Bisantrene is an anthracene derivative, which is much less cardiotoxic than the commonly used anthracyclines, including daunorubicin and doxorubicin. The antineoplastic activity of these agents, including bisantrene, is primarily attributed to their inhibition of topoisomerase II-mediated relaxation of the DNA supercoil torsion, which prevents DNA replication and cancer cell proliferation. Bisantrene inhibits the fat mass and obesity-associated protein (FTO), an RNA N6-methyladenosine (m6A) demethylase, and suppresses self-renewal of leukemia stem/initiating cells, further emphasizing its pharmacological and clinical value in therapy for AML as well as other cancers[3]. Prior clinical studies on the efficacy of bisantrene as a salvage drug led to its approval for treating AML in France in 1990. In a recent phase II study, bisantrene showed low toxicity in relapsed/refractory AML patients with an overall response rate of 40%.
Phlebitis and thrombosis of peripheral veins often occur at the bisantrene administration site. Rybak carefully studied the effect of bisantrene on platelet function and found that, in clinically achievable levels, bisantrene possesses a potent, rapid inhibitory activity against human platelets in vitro. Bisantrene impairs platelet aggregation to potent platelet agonists, including collagen, arachidonic acid, epinephrine, and calcium ionophore. Bisantrene does not appear to affect platelet cyclooxygenase. These effects may have a role in the phlebitis associated with bisantrene. In addition, platelets may play a role in tumor growth and metastasis, and the platelet inhibitory effect of bisantrene may be potentially significant in chemotherapy.
Metabolism
The metabolism of bisantrene, a new anthracene anticancer agent active in the treatment of disseminated breast cancer, was studied in vitro using rat liver S9 preparations and in vivo in patients receiving the drug as a treatment for their cancers. 14C-ring labeled bisantrene (248 mCi/40 rag) plus cold bisantrene were administered IV to cancer patients (260-340 mg/m2). Fractional urine samples were collected at various intervals up to 120 h after drug administration and analyzed by HPLC. The percent of total 14C excreted as unchanged parent drug per ml urine ranged from 37 to 79% in the 0 to 24 h samples[4]. In addition, research has found that the metabolic process involves an oxidative reduction, and there were at least 3 polar metabolites.
Cytotoxicity
Bisantrene has cytotoxic effects on dividing and non-dividing cells in vitro, suggesting the drug may be effective against low-growth fraction tumors. Bisantrene does produce inhibitory effects on antibody response to sheep red cells, which are not as severe as those experienced with other cytotoxic agents. When given intraperitoneally, it activates peritoneal exudate cells and putative macrophages, which subsequently inhibit the growth of murine tumors[5]. The cytostatic activity of the bisantrene-activated peritoneal exudate cells is profound and may account for the modest antibacterial effects observed in mice. It may also contribute to the antitumor effects of this drug.
References
[1] M E Rybak, T Griffin, B Badstubner. “The effects of bisantrene on human platelets.” Investigational New Drugs 4 2 (1986): 119–25.
[2] Jonathan Canaani. “A phase II study of bisantrene in patients with relapsed/refractory acute myeloid leukemia.” European Journal of Haematology 106 2 (2020): 260–266.
[3] Benigno C Valdez. “Enhanced cytotoxicity of bisantrene when combined with venetoclax, panobinostat, decitabine and olaparib in acute myeloid leukemia cells.” Leukemia & Lymphoma (2022): 1634–1644.
[4] Y M Peng, T P Davis, D S Alberts. “In vivo and in vitro metabolism of the new anticancer drug bisantrene.” Cancer Chemotherapy and Pharmacology 14 1 (1985): 15–20.
[5] Charles A. Coltman Jr, C. Kent Osborne. “Bisantrene, biological and clinical effects.” Cancer treatment reviews 11 4 (1984): Pages 285-288.
See also
Lastest Price from Bisantrene manufacturers

US $1.00/KG2024-10-22
- CAS:
- 78186-34-2
- Min. Order:
- 1KG
- Purity:
- MIn98%HPLC/LC
- Supply Ability:
- 100KGS
US $1.10/g2021-07-03
- CAS:
- 78186-34-2
- Min. Order:
- 1g
- Purity:
- 99.9%
- Supply Ability:
- 100 Tons Min