The application of Hexamethyldisilane
Introduction
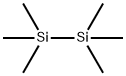
Hexamethyldisilane (Si2C6H18) is also known as Me3SiSiMe3. This organosilane is a hygroscopic colourless and odourless liquid which absorbs in the far UV region. It is constituted by silicon atoms bound to hydrocarbon groups as the methyl group. At room temperature and atmospheric pressure, it is found in the liquid state, but it reaches a vapour phase at low pressures. It can be easily synthesized by the reaction of chloroticmethyl silane with an alkali metal in organic solvents[1].
Uses
In organic chemistry, it is often used as an alternative to chlorotrimethylsilane or hexamethyldisilazane silylating agents. Hexamethyldisilane also promotes reduction and can act as a co-catalyst. It was recently revealed to be a key reagent in the anomerization of carbohydrates. Additionally, hexamethyldisilane can be a valuable starting material for synthesising silasubstituted organic and biologically active molecules. In the semiconductor industry, this oligoorganosilane is a precursor in the Chemical Vapor Deposition process.
Reduction of aromatic nitro compounds with hexamethyldisilane and fluoride ion in THF at 24°C gives the corresponding azo- and azoxy compounds in high yields. Hexamethyl-disilane converts commercial tetrabutylammoniumfluoride-dihydrate into a highly reactive catalyst[2].
Reaction profile
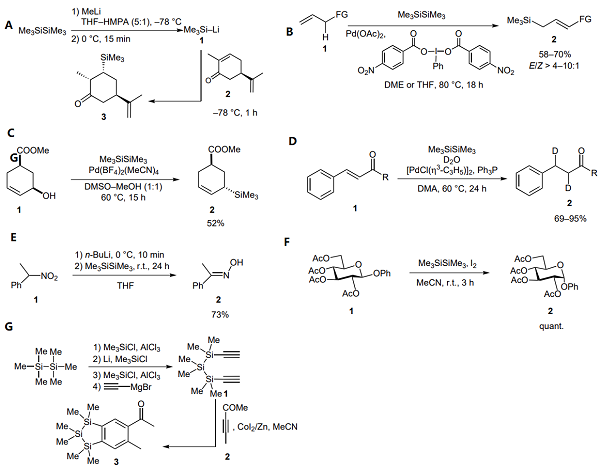
(A) Hexamethyldisilane as a Source of Trimethylsilylanion: In the stereoselective synthesis of 3-trimethylsilyldihydrocarvone developed by Blay et al., hexamethyldisilane is used as a precursor of the nucleophilic species 1. The Michael addition of Me3SiLi on (R)-(–)-carvone 2 proceeds in a very high yield (91%). This optimized procedure for cleavage a Si–Si bond is carried out under an inert atmosphere, using hexamethyl phosphoric triamide (HMPA) in a specific proportion with three temperature ranges[3].
(B) New C–C and C–Bond Activation: Rhodium-catalyzed silylation of the C–CN bond from arylnitriles and palladium-catalyzed allylic C–H silylation have been performed with hexamethyldisilane assistance. From the allylic substrates 1, excellent regio- and good stereoselectivities were observed with a high level of functional group tolerance.
(C) A Useful Allylic C–OH Activation: Szabó and co-workers described an inexpensive procedure for the regioselective synthesis of allylsilanes from allylic alcohols under palladium catalysis. With hexamethyldisilane, the silylation proceeds smoothly under mild conditions (50–60 °C, without an inert atmosphere, with no addition of acid or additive). Diverse functional groups (hydroxy-, methoxy- and nitrophenyl, ether and ester) and cyclic scaffolds like 1 are tolerated.
(D) Hexamethyldisilane as Co-Catalyst: Hexamethyldisilane promotes the reduction of alkynes and enones 1, desulfonation and Mizoroki–Heck-type coupling of arylnitriles to vinylsilanes. In these metal-catalyzed reactions, the disilane acts as a co-catalyst in generating the active catalytic species or by regenerating palladium or rhodium catalysts.
(E) Hexamethyldisilane as a Reducing Agent: Under mild conditions, nitroalkanes can be mono-deoxygenated to ketoximes by treatment of the corresponding nitronate anions with hexamethyldisilane. The cleaved disilane reacts as a ‘counterattack reagent’ by successive nucleophilic attacks of the resulting Me3Si– and Me3SiO– species. Bis(trimethylsilyl)ether is generated as a side product. For instance, N-(phenylethylidene)hydroxylamine 2 is formed in good yield (73%).
(F) Hexamethyldisilane/I2 as a Precursor of TMSI: Malik et al. demonstrated that the hexamethyldisilane–iodine system mediated anomerization of alkyl glycoside in quantitative yield. Indeed, the in situ formation of Me3SiI species leads to the anomerization step by ring opening of a silylated carbohydrate intermediate.
(G) Hexamethyldisilane as a Silicon Backbone: Starting from hexamethyldisilane, Tacke and co-workers build silicon analogues of biologically active molecules.16 The odorant 1,2,3- trisilaindane 3 is synthesized via a multistep procedure including two chlorodemethylation steps to prepare a dichlorotrisilane intermediate. Subsequent nucleophilic substitutions with ethynylmagnesium bromide lead to a trisiladiyne 1 engaged in a cobaltcatalyzed cycloaddition with the pent-3-yn-2-one 2. Notably, the trisila-phantolide derivative 3 displays a creamy-lactonic odor in contrast with the musk odor of its carbon analogue.
References:
[1] NEILETH S. FIGUEROA . Synthesis and characterization of hexamethyldisilane films deposited on stainless steel by plasma-enhanced chemical vapour deposition[J]. Surface & Coatings Technology, 2020, 404. DOI:10.1016/j.surfcoat.2020.126443.[2] VORBRUGGEN HELMUT K K. Reduction of aromatic nitro groups with hexamethyldisilane: Reactions with hexamethyldisilane and fluoride ion-II[J]. Tetrahedron Letters, 1984, 25 1: 1259-1262. DOI:10.1016/S0040-4039(01)80128-3.
[3] GIROS A. Hexamethyldisilane[J]. Synlett, 2012, 34 13. DOI:10.1055/s-0031-1290278.
You may like
Lastest Price from Hexamethyldisilane manufacturers
US $10.00/KG2021-03-18
- CAS:
- 1450-14-2
- Min. Order:
- 1KG
- Purity:
- 99.99
- Supply Ability:
- 10Ton

US $30.00/KG2020-07-05
- CAS:
- 1450-14-2
- Min. Order:
- 1KG
- Purity:
- min99%
- Supply Ability:
- 10MT/month