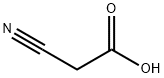
The application of Cyanoacetic acid in synthesis
Cyanoacetic acid is an organic compound with two functional groups, a nitrile NC and a carboxylic acid. It is a precursor to cyanoacrylates, components of adhesives [1]. Cyanoacetic acid is a versatile intermediate in the preparation of chemicals. It is a precursor to synthetic caffeine via the intermediacy of theophylline and it is a building block for many drugs, including dextromethorphan, amiloride, sulfadimethoxine, and allopurinol.
Application
Cyanoacetic acid is the raw materials for prepare N-tert butyl malonic acid methyl ester, which is used in pharmaceutical industry and dyeing industry (scheme 1).
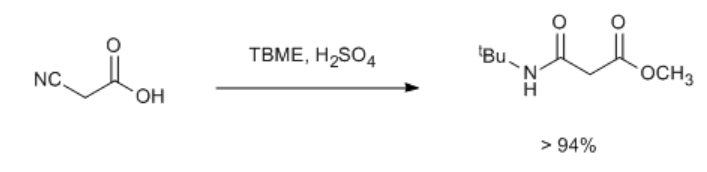
Scheme 1 The preparation of N-tert butyl malonic acid methyl ester
To a well-stirred mixture of cyanoacetic acid in tert butyl methyl ether (TBME) at about 10ºC was added sulfuric acid in 10 min time [2]. The mixture was then stirred at room temperature for 10–12 h and the reaction mixture was added to 20% sodium carbonate solution. After being extracted in TBME or dichloromethane, the combined organic layer was washed with water, and dried over sodium sulfate and concentrated under reduced pressure to obtain the product (94–98%).
It was also used in the preparation of a panchromatic dye for dye-sensitized solar cells. Sara and team studied optical and photovoltaic properties of thieno[3,2‑b]thiophene-based organic dyes for dye-sensitized solar cells.
Dye-sensitized solar cells (DSSCs) involving sensitizers adsorbed onto nanocrystalline TiO2 electrodes are of major interest because of their high incident solar light-to-electricity conversion efficiency, low cost, low maintenance, and high stability. Metal-free organic dyes have great advantages as sensitizers because of high molar extinction coefficient, simple and low cost synthesis and purification, and diverse possibilities for tuning the photophysical and electrochemical properties. The acceptor groups strongly influence the photovoltaic properties of the DSSC as they are key to electron injection from the dye molecule to the conduction band (CB) of the semiconductor film; they are also responsible for anchoring the sensitizing dye to the surface of the semiconductor. The most common anchoring group involves the carboxylic acid (−COOH) because of its relative stability, ease of synthesis, strong binding, and good electron communication between the dye and the surface, forming an ester linkage with TiO2 [3]. This group is normally used in the form of cyanoacetic acid.
Scheme 2. Reagents and Conditions: (a) 1,2-Dimethoxyethane, Pd(PPh3)4, N2, EtOH, and Na2CO3; (b) 2-Cyanoacetic Acid, Piperidine, and EtOH; (c) Rhodanine-3-acetic Acid, Piperidine, and EtOH.
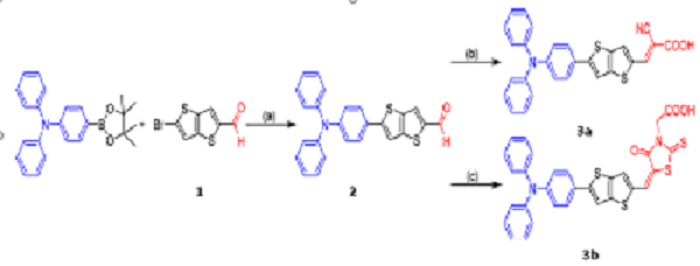
As shown in scheme 2, the dyes were obtained in moderate-to-excellent yields by the Suzuki−Miyaura coupling reaction, followed by Knoevenagel condensation. Compound 3a, bearing cyanoacetic acid as the acceptor/anchoring group, exhibits a better conversion efficiency in DSSCs.
References
[1] https://en.wikipedia.org/wiki/Cyanoacetic_acid
[2] Ramesha Ramakrishna, Esterification and Ritter reaction in one pot of cyanoacetic acid; N-tert butyl malonic acid methyl ester, Chemspider, synthetic pages, Jan 23, 2012, 532
[3] Sara S. M. Fernandes, etc., Optical and Photovoltaic Properties of Thieno[3,2‑b]thiophene-Based Push−Pull Organic Dyes with Different Anchoring Groups for Dye-Sensitized Solar Cells, ACS Omega 2017, 2, 9268−9279
You may like
See also
Lastest Price from Cyanoacetic acid manufacturers

US $10.00/KG2025-04-21
- CAS:
- 372-09-8
- Min. Order:
- 100KG
- Purity:
- 99%
- Supply Ability:
- 100 mt

US $0.00-0.00/Kg2025-04-21
- CAS:
- 372-09-8
- Min. Order:
- 1Kg
- Purity:
- 99.99%
- Supply Ability:
- 20 tons