What is the antioxidant activity of Caffeic acid during medication use?
Caffeic acid (CA, 3,4-dihydroxy-cinnamic acid) is an organic compound that is classified as a hydroxycinnamic acid. This yellow solid consists of both phenolic and acrylic functional groups, and it is found in most fruit, e.g., kiwis [1]. Caffeic acid is a type of polyphenol, a class of micronutrients known for their antioxidant properties. The nutrient is claimed to have many health benefits, including anti-inflammatory, anticancer, and antiviral abilities [2].
Antioxidants are compounds that inhibit or reduce the effects triggered by free radicals and oxidizing compounds. The phenylpropanoids act as antioxidants by eliminating oxygen free radicals and chelating pro-oxidant metal ions, especially iron. The hydroxyl groups of these molecules confer antioxidant activity, but they are not the only factors in determining the potency of their activities. There is a single hydroxyl group para-substituted in an aromatic ring that is linked to a side chain conjugate. This substitution allows the phenoxy radical free electrons to become delocalized over the entire molecule, and therefore stable. The ortho substitution to the methoxy group, an electron donor, is also a contributing factor to the stability of the phenoxy radical and thus increases the antioxidant efficiency. The presence of a second hydroxyl group in the ortho position, besides the para position, is known to increase the antioxidant activity due to an additional resonance stabilization and formation of o-quinone [3].

The anticarcinogenic properties of CA have attracted the attention of the scientific community. Studies have shown that the consumption of CA-rich foods leads to a protective effect against carcinogenesis by preventing the formation of nitro compounds (nitrosamines and nitrosamides) that are the main inducers of this pathology. The anti-carcinogenic action of CA is mainly associated with its antioxidant and pro-oxidant capacities, and this is attributed to its chemical structure. Firstly, the presence of free phenolic hydroxyls (ortho-dihydroxyl) makes it possible to reduce the enthalpy of OH-bond dissociation and increase the transfer rate of H atoms for peroxyl radicals, as well as their number and position on the phenyl ring (catechol group). Also, the presence of a double bond in the carbon chain (the unsaturated side chain 2,3 double bond) increases the stability of the phenolic radical. These chemical factors associated with the CA molecule enable the elimination of free radicals, preventing the production of ROS (reactive oxygen species) as well as the induction of DNA oxidation of cancer cells present in various types of cancer, such as Hepatocarcinoma (HCC).
The following example shows how does CA provide potent antioxidant capacity that prevents the production of ROS as treating HCC [4], and CA acts as a primary and a secondary antioxidant (mixed antioxidant). As a primary antioxidant, it acts by interrupting the formation of free radicals by inhibiting chain reactions with another molecule. This process occurs when CA donates electrons or hydrogen to free radicals, converting them into thermodynamically stable products. These products present greater stability due to the electron delocalised in the aromatic ring of the CA (resonance effect). In contrast, as a secondary antioxidant (prooxidant), it acts as a chelating agent. It forms complexes with metals (mainly iron and copper), inhibiting the decomposition of peroxides, reducing the formation of free radicals and their attack on lipids, amino acids of proteins, double bonding of polyunsaturated fatty acids and bases of DNA, thus avoiding the formation of lesions and loss of cellular integrity. CA has a great potential for reducing metals due to its structural chemical characteristics; the compound is susceptible to auto-oxidation and also the oxidation caused by the other biological agent. The molecular structure of CA, containing a catechol group with an α,β-unsaturated carboxylic acid chain, is responsible for its interaction with various types of oxidizing radicals. The o-dihydroxylated structure is where the o-quinone group is produced after the donation of an electron. The double side binding of o-quinone conjugated to the catechol group causes a delocalisation of electrons, increasing the stability of the o-quinone radical and the antioxidant activity of CA (scheme 1).
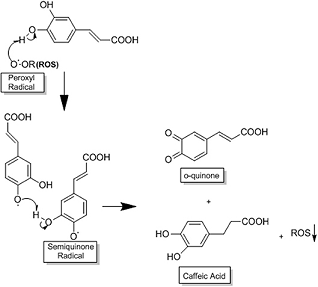
Scheme 1 The antioxidant process of CA
References
[1] https://en.wikipedia.org/wiki/Caffeic_acid
[2] https://www.healthline.com/health/caffeic-acid
[3] C. Magnani, etc., Caffeic acid: a review of its potential use in medications and cosmetics, Anal. Methods, 2014,6, 3203-3210.
[4] Kaio Murilo Monteiro Espíndola etc., Chemical and Pharmacological Aspects of Caffeic Acid and Its Activity in Hepatocarcinoma, Front. Oncol., 21 June 2019, 1-10,
[5] https://pubchem.ncbi.nlm.nih.gov/compound/689043
[6]http://www.chemspider.com/Chemical-Structure.2423.html?rid=a06f6655-b1db-49e7-8226-10804709107c&page_num=0
Related articles And Qustion
See also
Lastest Price from Caffeic acid manufacturers

US $0.00/kg2025-09-28
- CAS:
- 331-39-5
- Min. Order:
- 1kg
- Purity:
- 99%
- Supply Ability:
- 1

US $0.00/kg2025-04-27
- CAS:
- 331-39-5
- Min. Order:
- 1kg
- Purity:
- 0.99
- Supply Ability:
- 1000kg