Tacrolimus: pharmacokinetics, mechanism of action clinical applications and side effects
General Description
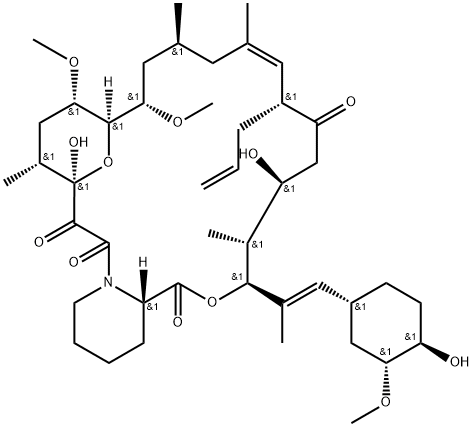
Tacrolimus is an immunosuppressive medication used to prevent organ rejection in transplant patients. It inhibits T-lymphocyte activity by binding to FKBP, blocking signal transduction and calcineurin inhibition. This reduces the risk of rejection. Tacrolimus also affects B-lymphocytes and natural killer cells. Side effects include renal toxicity, hypertension, diabetes, tremors, headache, and fatigue. Close monitoring is necessary. Tacrolimus is available as oral tablets and a topical ointment for other conditions like atopic dermatitis.

Figure 1. Capsules of tacrolimus
Pharmacokinetics
Tacrolimus is an immunosuppressive medication with distinct pharmacokinetic characteristics. It has a low average bioavailability of 25%, but this can vary greatly among individuals. Once in the bloodstream, Tacrolimus binds to red blood cells, but only the dissociated portion can enter the lymphatic system and have its primary effect. Absorption of Tacrolimus in the small intestine and liver is hindered by the first pass effect and pumping action of the small intestine. The activity of enzymes and transporters, such as CYP3A and P-gp, plays a significant role in Tacrolimus absorption and distribution. Metabolism of Tacrolimus primarily occurs in the liver, while pre-systemic biotransformation happens in the small intestine. The kidney also contributes to metabolism, primarily through CYP3A5. Tacrolimus has a relatively low total body clearance and a variable half-life of 4 to 41 hours, averaging around 12 hours. Most Tacrolimus metabolites are excreted through bile, with only a small proportion eliminated via urine and feces. Transporters like P-gp facilitate the excretion of Tacrolimus and its metabolites in various organs, including the intestines, kidneys, and liver. 1
Mechanism of action
Tacrolimus works by binding to the immunophilin protein FK-binding protein (FKBP), which results in the formation of a complex that inhibits the signal transduction of T-lymphocytes. This inhibition prevents the T-lymphocytes from rejecting the transplanted organ, thereby reducing the risk of organ rejection. Tacrolimus also has other effects that contribute to its immunosuppressive activity. It inhibits calcineurin, a phosphatase enzyme that plays a role in T-lymphocyte activation and proliferation. By inhibiting calcineurin, tacrolimus blocks the activation of T-lymphocytes and subsequent proliferation of these cells. This blockage helps to suppress the immune system and reduce the risk of organ rejection. In addition to its effect on T-lymphocytes, tacrolimus also affects other immune cells, including B-lymphocytes and natural killer cells. 2
Clinical applications
Tacrolimus is a macrolide immunosuppressant drug that is used to prevent organ rejection in transplant patients. It is approved for use in the United States under the brand name Prograf. The drug exerts its effect by inhibiting the activity of T-lymphocytes, which are responsible for rejecting transplanted organs. By inhibiting these cells, tacrolimus helps to prevent organ rejection and extends the survival of the transplanted organ. In addition to its use in organ transplantation, tacrolimus is also used off-label to treat various autoimmune diseases and other medical conditions. These include atopic dermatitis, psoriasis, and Chron's disease. In these diseases, tacrolimus helps to suppress the immune system and reduce the inflammation and symptoms associated with these conditions. Tacrolimus is available as oral tablets in doses of 0.5 mg, 1 mg, 2 mg, 5 mg, and 10 mg. It is also available as a 0.03% topical ointment that can be applied directly to the affected area of the skin. 3
Side effects
Tacrolimus can cause a range of side effects. Common ones include renal toxicity, hypertension, diabetes mellitus, tremors, headache, and fatigue. Renal toxicity can lead to kidney failure, necessitating dialysis. Hypertension affects about 50% of patients and may require anti-hypertensive treatment. Diabetes mellitus is reported in up to 40% of patients, potentially requiring insulin therapy. Tremors, headache, and fatigue affect about 30% and 20% of patients, respectively, potentially warranting dose adjustments or discontinuation. Tacrolimus also carries risks of heart disease, infections, and cancer. Close monitoring and prompt management of side effects are crucial for patients on tacrolimus. 4
Reference
1. Yu M, Liu M, Zhang W, Ming Y. Pharmacokinetics, Pharmacodynamics and Pharmacogenetics of Tacrolimus in Kidney Transplantation. Curr Drug Metab, 2018, 19(6):513-522.
2. Udomkarnjananun S, Francke MI, Dieterich M, van De Velde D, Litjens NHR, Boer K, De Winter BCM, Baan CC, Hesselink DA. P-glycoprotein, FK-binding Protein-12, and the Intracellular Tacrolimus Concentration in T-lymphocytes and Monocytes of Kidney Transplant Recipients. Transplantation. 2023, 107(2):382-391.
3. Zuo KJ, Saffari TM, Chan K, Shin AY, Borschel GH. Systemic and Local FK506 (Tacrolimus) and its Application in Peripheral Nerve Surgery. J Hand Surg Am, 2020, 45(8):759-765.
4. Weiler N, Thrun I, Eberlin M, Foltys D, Heise M, Hoppe-Lotichius M, Zimmermann T, Kraemer I, Otto G. Tacrolimus effects and side effects after liver transplantation: is there a difference between immediate and extended release? Transplant Proc, 2013, (6):2321-2325.
Related articles And Qustion
Lastest Price from Tacrolimus manufacturers

US $0.00-0.00/g2025-11-28
- CAS:
- 104987-11-3
- Min. Order:
- 1g
- Purity:
- 0.98
- Supply Ability:
- as required

US $5.00-0.50/KG2025-05-09
- CAS:
- 104987-11-3
- Min. Order:
- 1KG
- Purity:
- 99% hplc
- Supply Ability:
- 500TONS