Synthetic pathway of Sofpironium Bromide
Synthesis of Sofpironium Bromide
Sofpironium Bromide is prepared from two intermediates, α-hydroxyacid and pyrrolidine, by coupling and other reactions. The steps are as follows:
Step 1: Preparation of α-hydroxyacid for Sofpironium Bromide
Pivaldehyde acetonide protection of 103 preceded lithiation of 104 and quenching with cyclopentyl bromide to furnish dioxolanone 105, which was converted to α-hydroxyacid 106 upon treatment with base and pH adjustment.

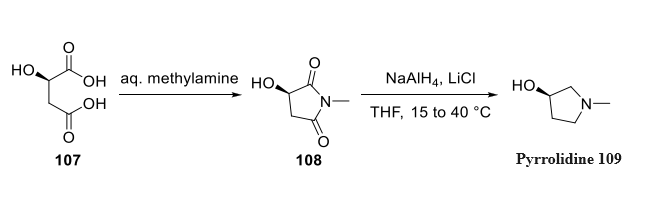
Step 2: Preparation of Sofpironium Pyrrolidine
Subjecting (R)-malic acid (107) to methyl amine formed hydroxysuccinimide 108, making way for carbonyl group reduction via aluminum hydride to furnish pyrrolidine 109. Although no yields were provided for the reduction protocol, an explicit synthesis of this intermediate has been disclosed in a recent patent application from Kaken Pharmaceuticals.
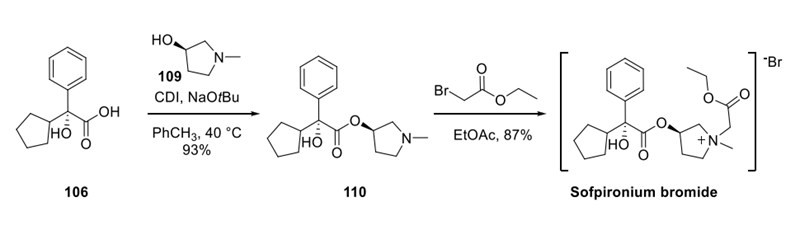
Step 3: Final Assembly of Sofpironium Bromide
To complete the synthesis of sofpironium bromide, acid 106 and alcohol 109 were coupled to form ester 110 using CDI. Alkylation with methyl bromoacetate furnished sofpironium bromide, which was isolated as a crystalline solid existing as several interchangeable crystalline forms.