Synthesis of Isothiazole
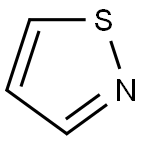
Isothiazole (1,2-thiazoles) is a five-membered heteroaromatic and considered to be derived from thiophene in which the second position is occupied by a nitrogen atom. Various penicillins and cephalosporins having an isothiazole ring system have shown considerable antibacterial efficacy against Gram-positive and Gram-negative bacteria. Many compounds with an isothiazole scaffold demonstrated a wide range of biological profiles, including antiinflammatory, antithrombotic, and anticonvulsive agents. Some of these molecules have been efficiently used in the treatment of Alzheimer’s disease. It has been found that isothiazole-based compounds show synergistic effects when used with other biocidal compounds. These molecules also act as herbicides and in combination with other herbicidal molecules they often enhance their efficacy. In addition, isothiazole derivatives have applications in color photography because they have been used for the stabilization of photomaterials. Some of the important compounds of this class are used as drugs and in clinical trials as mentioned in the following diagram.
Physical Properties
Isothiazole is a mobile, colorless liquid, with a bp of 114.1°C, a density of 1.352, and smells like pyridine. It is sparingly soluble in water. Isothiazole is a good solvent for many organic substances but should be used with caution because it is more toxic than pyridine. The more substituted isothiazoles are usually far less toxic.
Synthesis
A number of synthetic strategies are available for the construction of a variety of isothiazoles. However, some important synthetic protocols are described next.
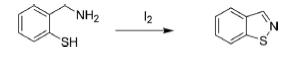
1. By Oxidation of 5-Amino-1,2-Benzoisothiazole
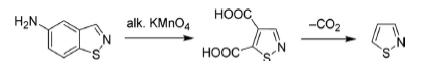
The parental isothiazole was initially prepared by the oxidation of 5-amino-1,2-benzoisothiazole in the presence of an alkaline solution of KMnO4 followed by decarboxylation of resulting isothiazole-4,5dicarboxylic acid.
2. Oxidative Cyclization of Acrylic Acid Amides
In the presence of oxidants, various α,β-unsaturated thiocarboxylic acid amides undergo cyclization to form a wide range of 5-aminoisothiazole derivatives.

Lastest Price from Isothiazole manufacturers

US $0.00/KG2025-04-15
- CAS:
- 288-16-4
- Min. Order:
- 1KG
- Purity:
- 99%
- Supply Ability:
- 500000kg