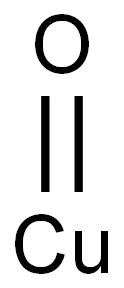Synthesis of CuO Nanoparticles
Cupric oxide is an inorganic chemical compound composed of cuprous ion and oxide ion. Cupric cuprous are the two forms of copper ions. Copper exists in two types of oxide, the one is with a higher oxidation state and another one is with a lower oxidation state, cupric oxide and cuprous oxide respectively.

Uses
Used in antifouling paints for boat and ship bottoms; it is an effective control over corrosion.
Used in paints for glass and porcelain.
Used as a p-type semiconductor material that was used to make photocells for light meters and fabricate rectifiers.
Used as a fungicide and seed dressing.
Difference between CuO and Cu2O
Cu2O is obtained by oxidizing copper metal or reducing sulfur oxide copper(II) solutions, while CuO is obtained by pyrometallurgical methods used to remove copper from ores. Most of the preservatives in wood are made from copper. This is often used as a pigment to shape various glazes.
Synthesis of CuO Nanoparticles
CuO nanostructures were synthesized by precipitation method using copper chloride (CuCl2) and copper nitrate (Cu(NO3)2.3H2O). First, each precursor was dissolved in 100 ml deionized water to form 0.1 M concentration. NaOH solution (0.1 M) was slowly dropped under vigorous stirring until pH reached to 14. Black precipitates were obtained and repeatedly washed by deionized water and absolute ethanol for several times till pH reached 7. Subsequently, the washed precipitates were dried at 80 °C for 16 h. Finally, the precursors were calcined at 500 °C for 4 h. investigated by X-Ray Diffractrometry (XRD). The morphology was monitored by scanning electron microscope (SEM). Chemical properties were investigated by Fourier transform infrared spectroscopy.
You may like
See also
Lastest Price from Cupric oxide manufacturers

US $1.00/KG2025-06-27
- CAS:
- 1317-38-0
- Min. Order:
- 1KG
- Purity:
- 99%
- Supply Ability:
- 10 mt

US $45.00/kg2025-04-21
- CAS:
- 1317-38-0
- Min. Order:
- 1kg
- Purity:
- 99%
- Supply Ability:
- 20 tons