Synthesis of 1-Bromobutane
1-Bromobutane is a primary alkyl halide (primary alkyl) and therefore it is produced from bimolecular nucleophilic substitution reactions (Sn2). Figure 1 shows the reaction for the synthesis of 1-bromobutane.
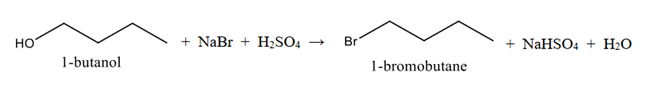
Figure 1. Global reaction for the synthesis of 1-bromobutane.
This halide is easily prepared by reacting butan-1-ol (primary alcohol) with sodium bromide solution and excess of concentrated sulfuric acid. The reaction between sodium bromide and sulphuric acid origins hydrobromic acid (Equation 1).
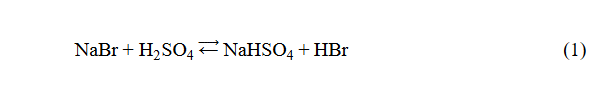
The use of excess of sulphuric acid allows to increase the degree of completion of the reaction. Also, the presence of a strong acid like sulphuric acid protonates the butan-1-ol, transforming the hydroxyl group (-OH) in a better leaving group, the water (H2O). The bromide ion from the hydrobromic acid reacts as nucleophile, occurring a substitution reaction. The mechanism of the reaction of the synthesis of 1-bromobutane is shown in Figure2.
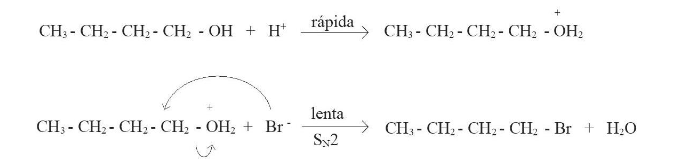
Figure 2. Mechanism of the reaction of the formation of 1-bromobutane.
The synthesis performed presents a complex experimental procedure, with several steps. After the mixture of the stoichiometric reagents, a reflux followed by a simple distillation are carried out, where some unwanted products can be separated such as, for example, sodium hydrogen sulphate and sulphuric acid. The liquid collected in the distillation is then washed with water, sulphuric acid and sodium hydroxide by liquid-liquid extraction to be isolated from other substances (but-1-ene, dibutyl ether and butan-1-ol that does not reacted). Finally, the product is dried with anhydrous calcium chloride and purified by simple distillation.
See also
Lastest Price from 1-Bromobutane manufacturers

US $0.00/KG2025-11-21
- CAS:
- 109-65-9
- Min. Order:
- 1KG
- Purity:
- ≥99%
- Supply Ability:
- 80 Tons/Month

US $10.00/kg2025-04-21
- CAS:
- 109-65-9
- Min. Order:
- 1kg
- Purity:
- 99%
- Supply Ability:
- 10 mt