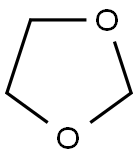
Synthesis of 1,3-Dioxolane
1,3-Dioxolane is a five-membered, nonplanar, fully saturated oxygen heterocycle with two oxygen atoms at the 1,3-positions of the cyclic system. It closely resembles THF because the methylene group at position 3 is replaced by an oxygen atom. In other words, it is a five-membered cyclic acetal.
1,3-Dioxolane derivatives are recognized as important motifs for the construction of numerous pharmacologically active molecules as antiviral, antifungal, anti-HIV, and adrenoreceptor antagonists.

Physical Properties
1,3-Dioxolane is a colorless liquid. It is fully miscible with water, ether, acetone, and THF. It readily dissolves waxes, plastics, fats, and oils, with a bp of 78°C. It acts as a base and forms salts with strong acids.
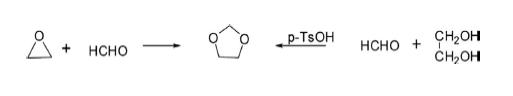
Synthesis of 1,3-Dioxolane
The parent 1,3-dioxolane has been obtained through condensation of ethylene glycol with formaldehyde in toluene using p-toluenesulfonic acid as catalyst. The same has also been obtained by the reaction of ethylene oxide with formaldehyde using SnCl4 or tetraethylammonium bromide as catalyst.
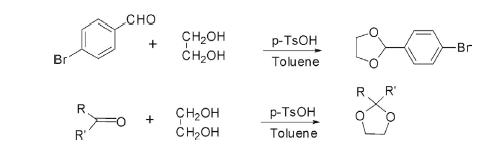
Acetalization or ketalization of aldehyde and ketones with ethylene glycol in the presence of p-toluenesulfonic acid forms mono- and disubstituted 1,3-dioxolanes separately.
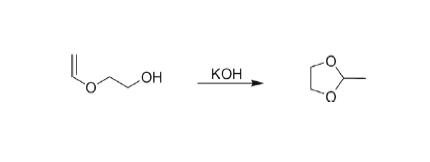
Synthesis of 2-methyl-1,3-dioxolane has been reported by heating vinyl ether with KOH.
You may like
Lastest Price from 1,3-Dioxolane manufacturers

US $100.00/KG2025-04-21
- CAS:
- 646-06-0
- Min. Order:
- 1KG
- Purity:
- 99%min
- Supply Ability:
- 200TON

US $10.70/KG2024-10-11
- CAS:
- 646-06-0
- Min. Order:
- 10KG
- Purity:
- 99%
- Supply Ability:
- 10000kg