The Role of Dolutegravir Sodium in Transforming HIV Care: From Mechanism of Action to Clinical Application
Dolutegravir Sodium Introduction
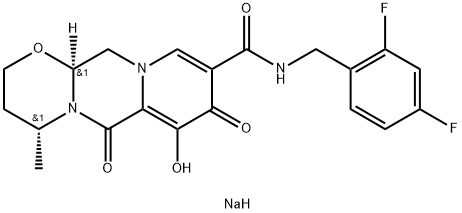
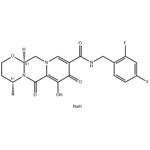
Dolutegravir sodium is a white to off-white powder that is soluble in water and classified as an integrase inhibitor. The development of this drug marked a significant milestone in anti-HIV therapy, with its approval by the United States Food and Drug Administration (FDA) first granted in 2013. The discovery of Dolutegravir was based on a comprehensive understanding of the role of integrase in the HIV replication cycle, where the enzyme is responsible for integrating viral DNA into the host cell genome, a crucial step for virus replication[1].

Figure 1: Characteristics of Dolutegravir sediment
Mechanism of Action
The mechanism of action of Dolutegravir sodium is a testament to its innovative approach to targeting HIV-1. This drug exerts its therapeutic effects by selectively inhibiting the integrase enzyme, a key protein required by HIV to replicate within human cells. The integrase enzyme plays a pivotal role in the HIV replication cycle by integrating the viral DNA into the DNA of the host cell, a process essential for the production of new virus particles. Dolutegravir sodium binds with high affinity to the active site of the integrase enzyme, preventing the viral DNA from integrating into the host cell genome. This interruption halts the replication cycle of the virus at a critical juncture, effectively preventing the production of new HIV particles.
What sets Dolutegravir apart from other anti-HIV medications is its unique chemical structure, which allows for a stronger and more enduring interaction with the integrase enzyme. This leads to a potent inhibition of the virus's ability to replicate, even in cases where the virus has developed resistance to other integrase inhibitors. Moreover, the binding efficiency of Dolutegravir reduces the likelihood of the virus developing resistance against it, making it an effective component of antiretroviral therapy over extended periods.
The efficacy of Dolutegravir inhibits a broad spectrum of HIV strains, including those resistant to other antiretroviral drugs, is another significant advantage. This broad-spectrum activity, combined with its high barrier to resistance, underscores the critical role of Dolutegravir in the management of HIV, offering a promising option for patients with limited treatment choices due to resistance issues.
Application
Dolutegravir sodium's application in the treatment of HIV-1 is both versatile and comprehensive. Its use as monotherapy, or more commonly in combination with other antiretroviral agents, forms the backbone of modern HIV therapy protocols. The drug's potency and high barrier to resistance make it a suitable option for a wide range of patients, including those newly diagnosed with HIV, as well as patients who have experienced virological failure with other regimens.
For adults, adolescents, and children living with HIV, Dolutegravir sodium offers a chance for effective disease management and a better quality of life. Its efficacy is not significantly affected by the presence of most resistance mutations, making it a robust option for individuals who have encountered resistance to first-line treatments. Furthermore, Dolutegravir's compatibility with other drugs allows it to be integrated into various therapeutic regimens, enhancing its utility in diverse patient populations.
Clinical guidelines across the globe have recognized the value of Dolutegravir sodium, recommending it as a first-line treatment option for HIV-1 infection. Its role in public health strategies aimed at controlling the HIV epidemic is increasingly significant, providing a reliable foundation for both initial and salvage therapy plans[2].
Side Effects
Like all medications, Dolutegravir sodium is not without side effects. Commonly reported adverse effects include insomnia, headache, and gastrointestinal issues such as nausea and diarrhea. Rare but serious side effects may include hypersensitivity reactions and liver toxicities. Strategies for managing and mitigating these side effects are critical for maintaining patient quality of life and adherence to treatment.
Storage
The proper storage of Dolutegravir sodium is essential for maintaining its efficacy. The drug should be stored at room temperature, away from light and moisture. Special attention must be given to the expiration date, and any expired or unused medication should be disposed of properly to prevent misuse.
References
[1]Dowers E, Zamora F, Barakat L A, et al. Dolutegravir/rilpivirine for the treatment of HIV-1 infection[J]. HIV/AIDS-Research and Palliative Care, 2018: 215-224.
[2] Zamora F J, Dowers E, Yasin F, et al. Dolutegravir and lamivudine combination for the treatment of HIV-1 infection[J]. HIV/AIDS-Research and Palliative Care, 2019: 255-263.
Related articles And Qustion
See also
Lastest Price from Dolutegravir sodium manufacturers
US $0.00/kg2025-11-25
- CAS:
- 1051375-19-9
- Min. Order:
- 1kg
- Purity:
- 98%
- Supply Ability:
- Customise

US $0.00/g2025-01-13
- CAS:
- 1051375-19-9
- Min. Order:
- 1g
- Purity:
- More Than 99%
- Supply Ability:
- 50kg/Month