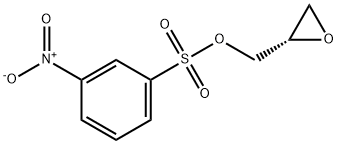
Synthesis and Applications of (S)-(+)-Glycidyl nosylate
(S)-(+)-Glycidyl nosylate, an off-white or brownish-yellow needle crystal at room temperature. (S)-(+)-Glycidyl nosylate is mainly used as an intermediate in organic synthesis and pharmaceutical chemistry, and is a key intermediate in the synthesis of drug molecule Landiolol hydrochloride. Landiolol hydrochloride is an ultrashort-acting and highly selective β1 receptor blocker, which mainly antagonizes β1 receptors in the heart and ameliorates tachycardic arrhythmias by inhibiting the increase of the heart beats caused by catecholamines.
Synthesis
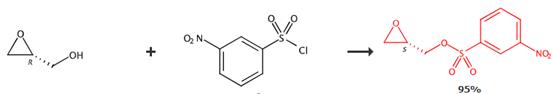
Figure1 Synthetic route of (S)-(+)-Glycidyl nosylate
Method 1:
Add 3-nitrobenzene-1-sulfonyl chloride (730 mg, 3.3 mmol) portionwise to a cooled solution of (S)-oxiran-2-ylmethanol (220 mg, 3.0 mmol) in anhydrous CH2Cl2 (10 mL) and triethylamine (1.5 mL, 10 mmol). Monitor the reaction by TLC. Quench the reaction mixture with ice. Extract the reaction mixture with CH2Cl2/water. Wash the organic layer with saturated aqueous NaHCO3.Dry the organic layer over Na2SO4.Purify the concentrate product by flash column chromatography. [1]
Method 2:
Suspend the solfonyl derivative (18.0 g, 81.0 mmol) in toluene (200 mL) and cooled to -40°C. Add Et3N (11.2 mL, 81.0 mmol) and epoxide derivative (5.46 g, 73.66 mmol). Stir the reaction for 17 hours before it is allowed to warm to -10°C. Stir the reaction for another 8 hours. Transfer the mixture to a separation funnel with EtOAc (200 mL) and sulfate buffer (250 mL). Separate the phases. Wash the organic layer with additional sulfate buffer (250 mL), sat. aq. NaHCO3 (250 mL) and brine (250 mL). Dry the crude over Na2SO4 and concentrate in vacuo to obtain the product. [2]
Applications
(S)-(+)-Glycidyl nosylate can be used as an intermediate in medicinal chemistry and organic synthesis, is a key intermediate in the synthesis of drug molecule Landiolol hydrochloride. Landiolol hydrochloride can be used for the emergency treatment of tachyarrhythmias (including atrial fibrillation, atrial flutter, sinus tachycardia) during surgery. Emergency treatment of tachyarrhythmias (including atrial flutter, atrial fibrillation, sinus tachycardia) under postoperative ambulatory monitoring of the circulation. In organic synthetic transformations, the ternary ring in the structure can be attacked by nucleophilic reagents to obtain ring-opening derivatives. In addition, the sulfonate group in the structure is a good leaving group and also the corresponding sulfonate can be substituted by the nucleophilic reagent.
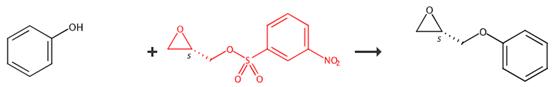
Figure2 Applications of (S)-(+)-Glycidyl nosylate
A solution of phenol (9.4 g, 100 mmol) and (2S)-(+)glycidyl 3-nitrobenzenesulfonate (25.9 g, 100 mmol) in 500 mL of acetone was treated with 3 equivalents of potassium carbonate (41.5 g, 300 mmol) and stirred at reflux for 1 day. The suspension was cooled to ambient temperature; the solid was filtered; and the filtrate concentrated to dryness. The residue was partitioned betw een methylene chloride and w ater and the aqueous layer was extracted with methylene chloride. The organic layers were combined and dried over magnesium sulfate and concentrated to give the title compound. Orange oil, yleld 15.0 g, 99%. [3]
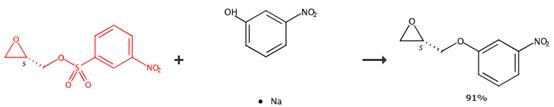
Figure3 Applications of (S)-(+)-Glycidyl nosylate
Add phenol (7.7 mmol) to a suspension of NaH (7.7 mmol) in anhydrous DMF (40 mL) kept at room temperature under N2atmosphere. When the reaction mixture appeared clear, add a solution of (S)-(+)-glycidyl nosylate (2 g, 7.7 mmol) in anhydrous DMF (40 mL) to the reaction mixture. Stir the reaction mixture at room temperature until completion. Add H2O to the reaction mixture. Extract the aqueous layer four times with EtOAc. Wash the combined extracts three times with a saturated aqueous Na2CO3. Evaporate the combined extracts several times with H2O. Dry the combined extracts over anhydrous Na2SO4. Remove the solvent under reduced pressure. Isolate the product (an oil) by chromatography (eluent: PE/EtOAc = 8:2) (249 mg, 83%).[4]
References
[1] Abdul-Hay, Samer et al ACS Medicinal Chemistry Letters, 2(9), 656-661; 2011
[2] Qu, Chun-Ping and Xu, Qing-Ling Latin American Journal of Pharmacy, 40(11), 2817-2820; 2021
[3] Hu, Baihua PCT Int. Appl., 2002006281, 24 Jan 2002
[4] Perrone, Maria Grazia et al ChemMedChem, 4(12), 2080-2097; 2009
See also
Lastest Price from (S)-(+)-Glycidyl nosylate manufacturers

US $0.00-0.00/KG2025-04-28
- CAS:
- 115314-14-2
- Min. Order:
- 1KG
- Purity:
- 98
- Supply Ability:
- 1KG 100KG 1MT

US $0.00/KG2025-04-21
- CAS:
- 115314-14-2
- Min. Order:
- 1KG
- Purity:
- 98%min
- Supply Ability:
- 30tons/month