Synthesis and application of α-Tocopherol
Background
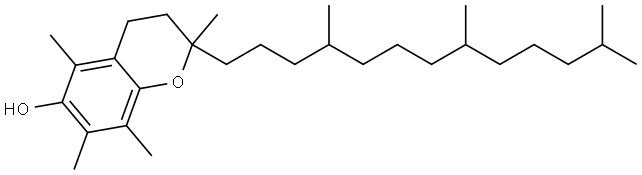
α-Tocopherol ((2R)-2,5,7,8-Tetramethyl-2-[(4R,8R)-4,8,12-trimethyltridecyl]-3,4-dihydro-2H-1-benzopyran-6-ol) is a type of vitamin E. It has E number "E307". Vitamin E exists in eight different forms, four tocopherols and four tocotrienols. All feature a chromane ring, with a hydroxyl group that can donate a hydrogen atom to reduce free radicals and a hydrophobic side chain which allows for penetration into biological membranes. Compared to the others, α-tocopherol is preferentially absorbed and accumulated in humans.
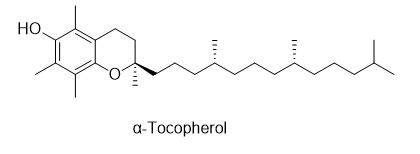

Vitamin E is found in a variety of tissues, being lipid-soluble, and taken up by the body in a wide variety of ways. The most prevalent form, α-tocopherol, is involved in molecular, cellular, biochemical processes closely related to overall lipoprotein and lipid homeostasis. Ongoing research is believed to be "critical for manipulation of vitamin E homeostasis in a variety of oxidative stress-related disease conditions in humans."[2] One of these disease conditions is the α-tocopherol role in the use by malaria parasites to protect themselves from the highly oxidative environment in erythrocytes. [3]
Stereoisomers
α-Tocopherol has three stereocenters, so it is a chiral molecule.[4] The eight stereoisomers of α-tocopherol differ in the configuration of these stereocenters. RRR-α-tocopherol is the natural one. [5] The older name of RRR-α-tocopherol is d-α-tocopherol, but this d/l naming should be no longer used, because whether l-α-tocopherol should mean SSS enantiomer or the SRR diastereomer is not clear, from historical reasons. The SRR may be named 2-epi-α-tocopherol, the diastereomeric mixture of RRR-α-tocopherol and 2-epi-α-tocopherol may be called 2-ambo-α-tocopherol (formerly named dl-α-tocopherol). The mixture of all eight diastereomers is called all-rac-α-tocopherol. [6]
One IU of tocopherol is defined as 2⁄3 milligram of RRR-α-tocopherol (formerly named d-α-tocopherol). 1 IU is also defined as 0.9 mg of an equal mix of the eight stereoisomers, which is a racemic mixture, all-rac-α-tocopheryl acetate. This mix of stereoisomers is often called dl-α-tocopheryl acetate. [7] Starting with May 2016, the IU unit is made obsolete, such that 1 mg of "Vitamin E" is 1 mg of d-alpha-tocopherol or 2 mg of DL-alpha-tocopherol. [8]
Preparation

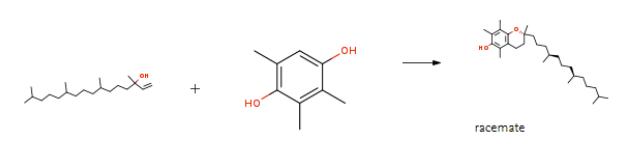
Scheme 2. The synthesis of racemate
There are many synthetic methods ofα-Tocopherol ((2R)-2,5,7,8-Tetramethyl-2-[(4R,8R)-4,8,12-trimethyltridecyl]-3,4-dihydro-2H-1-benzopyran-6-ol) reported in the literature, one of which and the specific method of synthesis are as follows .7.55 g (50 mmol) of trimethylhydroquinone (purity 99.7 %), 40 g of ethylene carbonate (or propylene carbonate) and 50 ml of heptane were introduced into a 200 ml four-necked flask equipped with a reflux condenser, a water separator, a mechanical stirrer and an argon gasification means and heated to reflux temperature (bath temperature 140 °C) under an argon atmosphere. After the addition of methane trisulphonate as an aqueous solution (for 0.05 mole % of CH (S03H) 3 based on the molar amount of subsequently added isophytol 4.23 mg = 391 UL of catalyst were used), 12.026 ml (33 mmol) of isophytol were added at a rate of 0.6 ml/minute. Thus the volume ratio of trimethylhydroquinone to isophytol was about 1.5: 1. Thereafter the heptane was distilled off and the mixture was heated to 125-130°C for 30 minutes, then cooled to 80°C. 50 ml of heptane were added to the ethylene carbonate phase. The reaction mixture was stirred for a further 10 minutes at 50°C. The heptane layer was then separated and evaporated under reduced pressure to give (ALL-RAC)- C-TOCOPHEROL as a viscous oil in a yield shown in the following Tables 1 and 2 in which EC signifies ethylene carbonate, PC signifies propylene carbonate and IP signifies isophytol.[9]

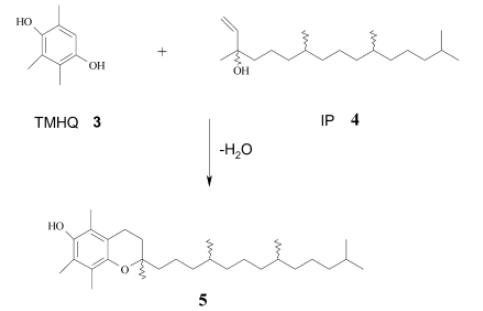
Scheme 2. The synthesis of (dl)-[α]-tocopherol 5 by the condensation of TMHQ 3 and IP 4
For synthesis of (DL)-[α]-tocopherol, in a typical procedure, 152 mg of TMHQ was dissolved in 10 mL of solvent (acetonitrile, hexane, toluene, DMC, or hexane:DMC [50:50]) in a two-necked flask. Then 50 mg of catalyst was added to this mixture. Once the temperature reached 100 °C, 0.4 mL of IP (TMHQ: IP molar ratio = 1:1) was added dropwise under stirring. The reaction was carried out under reflux conditions for 1 h. After the reaction was stopped, the catalyst was filtered off and the product separated from solvent under vacuum at 80 °C, as light-yellow–brown oil. Yields and purity were determined by HPLC analysis under following conditions: column, EC 125/4.6 NUCLEOSIL 120-5 C18; eluent, acetonitrile; flow rate, 0.8 mL/min; wavelength, 280 nm; volume sample, 15 μL. The compounds were identified with the aid of pure samples (standards). [10]
Application
Using DL-α-tocopherol as the lipophilic end of the surfactant, polyethylene glycol (polyoxyethylene ether, EO) chain. As the hydrophilic end, the nonionic surfactant DL-α-tocopherol methoxy polyethylene glycol succinate (DL-α-tocopherol methoxypolyethylene glycol succinates, referred to as TPGS-X-M (X=750, 1000, 1500, 1900, indicating the Use the relative molecular mass of polyethylene glycol monomethyl ether).

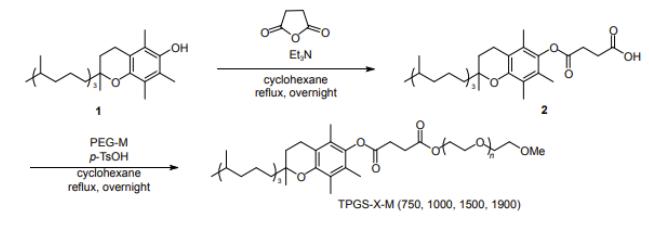
The method of synthesizing this active agent is as follows. Combine DL-α-tocopherol (1, 0.15 mol, 64.5 g) and butadiene Anhydride (0.225 mol, 22.5 g) in cyclohexane solution (300 mL) Reflux, using Dean-Stark trap to continuously separate the reaction products of water and stir overnight. Wait until the liquid level in the water separator no longer rises. When the time, it means that the reaction has ended. Add the right amount to the mixture water, extract 3 times with dichloromethane, hydrochloric acid (1 mol/L, 50 mL) wash 3 times, wash 2 times with water (30 mL), dry with anhydrous magnesium sulfate dry, use a rotary evaporator to remove the organic solvent to obtain a yellow liquid body compound 2, 81.7 g, yield 99%.
Reference
[1] Merck Index, 11th Edition, 9931.
[2] Rigotti A (2007). "Absorption, transport, and tissue delivery of vitamin E". Molecular Aspects of Medicine. 28 (5–6): 423–36. doi: 10.1016/j.mam.
[3] Shichiri M, Ishida N, Hagihara Y, Yoshida Y, Kume A, Suzuki H (2019). "Probucol induces the generation of lipid peroxidation products in erythrocytes and plasma of male cynomolgus macaques". Journal of Clinical Biochemistry and Nutrition. 64 (2): 129–142. doi:10.3164/jcbn.18-7.
[4] Jensen SK, Lauridsen C (2007). "Alpha-tocopherol stereoisomers". Vitamins and Hormones. 76: 281–308. doi:10.1016/S0083-6729(07)76010-7.
[5] Brigelius-Flohé R, Traber MG (July 1999). "Vitamin E: function and metabolism". FASEB Journal. 13 (10): 1145–55. doi:10.1096/fasebj.13.10.1145.
[6] IUPAC Nomenclature of Tocopherols and Related Compounds.
[7] "Composition of Foods Raw, Processed, Prepared USDA National Nutrient Database for Standard Reference, Release 20"
You may like
See also
Lastest Price from DL-α-Tocopherol manufacturers

US $0.00/KG2025-04-21
- CAS:
- 10191-41-0
- Min. Order:
- 1KG
- Purity:
- 98%min
- Supply Ability:
- 30tons/month

US $10.00/KG2025-04-21
- CAS:
- 10191-41-0
- Min. Order:
- 1KG
- Purity:
- 99%
- Supply Ability:
- 100 mt