Synthesis and Application of 2,4,6-Trichloroaniline
General description
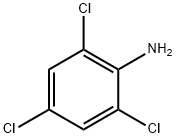
2,4,6-Trichloroaniline is a white to light yellow crystal, easily soluble in carbon disulfide, soluble in ethanol, diethyl ether, petroleum ether, insoluble in water. It's stimulating. Melting point 73-75℃, boiling point 262℃, water solubility < 0.1g /100 mL at 21.5℃. Used as raw materials for azo dyes, insecticides, fungicides, herbicides and basic fuchsin coupling agents for photography. White to light yellow crystal, easily soluble in carbon disulfide, soluble in ethanol, ether, petroleum ether, insoluble in water. It's stimulating. Melting point 73-75℃, boiling point 262℃, water solubility < 0.1g /100 mL at 21.5℃. Used as raw materials for azo dyes, insecticides, fungicides, herbicides and basic fuchsin coupling agents for photography.
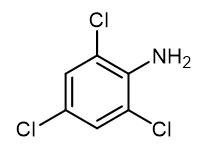
Fig. 1 The structure of 2,4,6-Trichloroaniline.
Synthetic routes
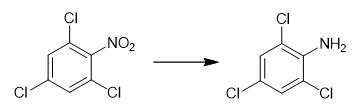
Fig. 2 The synthetic method 1 of 2,4,6-Trichloroaniline.
Charge substituted nitrobenzene (62 mg), molybdenum catalyst (Mo content: 5.0 wt%, 24.0 mg, the proportion of Mo to nitrobenzene is 2.5 mol%), THF (2.5 mL), H2O (2.5 mL) into a 50 mL stainless autoclave. Pressurize the mixture to 5.0 MPa H2 at room temperature. Heat the mixture to the 120 °C for 20 hours. After completion of the reaction, cool the autoclave to room temperature. Depressurize the mixture. Extract the result mixture with EtOAc. Remove the solvent of organic layer under reduced pressure. Purify the crude product by a silica gel chromatography using petroleum ether/ethyl acetate 6:1. 1H NMR (400 MHz, CDCl3): δ 7.19 (s, 2H), 4.43 (br, 2H). 13C NMR (100 MHz, CDCl3): δ 139.0, 127.6, 121.9, 119.7 [1].
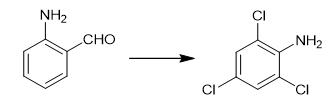
Fig. 3 The synthetic method 2 of 2,4,6-Trichloroaniline.
Take vanadium pentoxide (18 mol%, approximately 17 mg) in a 20 mL screw-capped reaction tube. Add a 2 mL pH (1.0) solution followed by 12 mmol (0.895 g) of KCl to the mixture. Add 0.5 mmol of 2-aminobezaldehyde in 1 mL of HCl to the mixture. Add 12 mmol (330 μL) of 30 % H2O2 to the contents of the reaction tube. Close the screw cap. Seal the screw cap with paraffin. Keep the reaction tube at room temperature with constant stirring. After 12 hours, add 50 mL of dichloromethane to the reaction mixture. Extract the organic component. Repeat the process. Combine the organic parts. Dry the organic parts over Na2SO4. Concentrate the organic parts under reduced pressure in a rotary evaporator. Purify the crude product for column chromatography (silica gel, 60-120 mesh) 5% ethyl acetate in petroleum ether as the eluent [2].
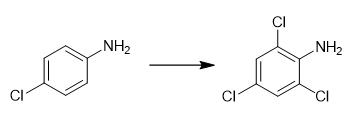
Fig. 4 The synthetic method 3 of 2,4,6-Trichloroaniline.
Add chlorinating reagent (1.2 equiv.) to a stirred solution of heterocycle (1.0 equiv.) in anhydrous DMF at room temperature. Stir the mixture for 12 h under air condition. Quench the resulting mixture with saturated aqueous NaHCO3, extract with ethyl acetate (15 mLx3). Dry the combine organic layer over anhydrous Na2SO4 and filter. Remove the solution by evaporation under reduced pressure. Purify the crude product by column chromatography on silica gel using petroleum ether/ethyl acetate as eluent to obtain the product [3].
Application
As the coupling agent
A new spectrophotometric method is described for the determination of propoxur (O-isopropoxy phenyl, methyl carbamate) and carbosulfan (2,3-dihydro-2,2-dimethylbenzofuran-7-yl (dibutyl-aminothio) methyl carbamate) by the diazotization-coupling reaction using 2,4,6-trichloroaniline as the coupling agent. The phenols of the insecticides are generated by alkaline hydrolysis. The absorption maximum of orange coloured species were 443 run and 440 run. The coloured species obey Beer's law in the concentration range 0-10 ppm [4].
Sorption and desorption behavior of chloroanilines
The bioavailability of pollutants, pesticides and/or their degradation products in soil depends on the strength of their sorption by the different soil components, particularly by the clay minerals. This study reports the sorption-desorption behavior of the environmentally hazardous industrial pollutants and certain pesticides degradation products, 3-chloroaniline, 3,4-dichloroaniline, 2,4,6-trichloroaniline, 4-chlorophenol, 2,4-dichlorophenol and 2,4,6-trichlorophenol on the reference clays kaolinite KGa-1 and Na-montmorillonite SWy-1. In batch studies, 2.0 g of clay were equilibrated with 100.0 mL solutions of each chemical at concentrations ranging from 10.0 to 200.0 mg/L. The uptake of the compounds was deduced from the results of HPLC-UV-Vis analysis. The lipophilic species were best retained by both clay materials. The most lipophilic chemical used in the study, 2,4,6-trichloroaniline, was also the most strongly retained, with sorption of up to 8 mg/g. In desorption experiments, which also relied on HPLC-UV-Vis technique, 2,4,6-trichloroaniline was the least desorbed from montmorillonite. However, on kaolinite all of the compounds under study were irreversibly retained. The experimental data have been modelled according to the Langmuir and Freundlich isotherms. A hypothesis is proposed concerning the sorption mechanism and potential applications of the findings in remediation strategies have been suggested [5].
References
[1] Yan S, Guo Y, Xiao L P, et al. Chemoselective Hydrogenation of Functionalized Nitroarenes into Anilines by Supported Molybdenum Catalysts[J]. ChemistrySelect, 2020, 5(24): 7249-7253.
[2] Rana S, Pandey B, Dey A, et al. A Doubly Biomimetic Synthetic Transformation: Catalytic Decarbonylation and Halogenation at Room Temperature by Vanadium Pentoxide[J]. ChemCatChem, 2016, 8(21): 3367-3374.
[3] Wang M, Zhang Y, Wang T, et al. Story of an age-old reagent: an electrophilic chlorination of arenes and heterocycles by 1-chloro-1, 2-benziodoxol-3-one[J]. Organic letters, 2016, 18(9): 1976-1979.
[4] Harikrishna V, Prasad B, Naidu N V S. Spectrophotometric determination of propoxur and carbosulfan with 2, 4, 6-trichloroaniline as the coupling agent[J]. Asian Journal of Chemistry, 2003, 15(2): 1013-1019.
[5] Polati S, Gosetti F, Gianotti V, et al. Sorption and desorption behavior of chloroanilines and chlorophenols on montmorillonite and kaolinite Part B Pesticides, food contaminants, and agricultural wastes[J]. 2006.
You may like
See also
Lastest Price from 2,4,6-Trichloroaniline manufacturers

US $50.00-20.00/KG2025-04-21
- CAS:
- 634-93-5
- Min. Order:
- 1KG
- Purity:
- 99%
- Supply Ability:
- 20 MT

US $8.00/kg2024-11-07
- CAS:
- 634-93-5
- Min. Order:
- 1kg
- Purity:
- 99%
- Supply Ability:
- 20000