Synthesis and application of 1-bromo-8-chloronaphthalene
Brief description
CAS: 20816-79-9
Molecular formula: C10H6BrCl
Molecular weight: 241.51
Color: White solid
Melting point: 87-88 °C
Boiling point: 150-160 °C (Press: 5-6 Torr)
Storage condition: Sealed in dry, Room Temperature
Density: 1.592±0.06 g/cm3 (Predicted)
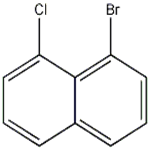
Fig. 1 The structure of 1-bromo-8-chloronaphthalene
Synthetic routes:
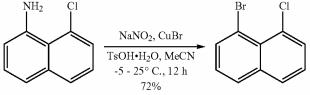
Fig. 2 The synthetic scheme 1 of 1-bromo-8-chloronaphthalene.
To a solution of 8-chloronaphthalen-1-amine (57 g, 320 mmol, 1 eq) and TsOH•H2O (219 g, 1.16 mol, 3.6 eq) in MeCN (1000 mL) was added a solution of NaNO2 (39.8 g, 577 mmol, 1.8 eq) and CuBr (138 g, 963 mmol, 29.3 mL, 3 eq) in H2O (120 mL) at -5 °C, then the reaction mixture was stirred at 25 °C for 12 hours. The reaction mixture was added saturated Na2SO3 solution (100 mL), stirred for 15 mins, and then extracted with ethyl acetate (3 x 1000 mL). The combined organic layers were washed with brine (500 mL), dried over Na2SO4, filtered and concentrated under reduced pressure to give a residue. The residue was purified by column chromatography (SiO2, Petroleum ether) to give 1-bromo-8-chloronaphthalene (56 g, 229 mmol, 72% yield, 99% purity). White solid; 1H NMR (400 MHz, chloroform-d): δ 7.93 (dd, J = 1.2, 7.6 Hz, 1H), 7.82 (dd, J = 1.2, 8.4, 1H), 7.79 (dd, J = 1.2, 8.4, 1H),7.67 (dd, J = 1.2, 7.6 Hz, 1H), 7.37 (t, J = 8.0 Hz, 1H), 7.28 (t, J= 8.0 Hz, 1H) [1].
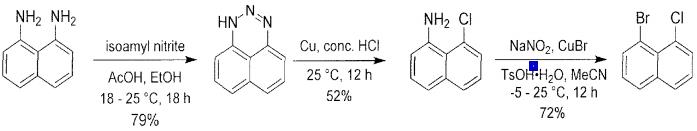
Fig. 3 The synthetic scheme 2 of 1-bromo-8-chloronaphthalene.
To a solution of 8-chloronaphthalen-1-amine (20.2 g, 114 mmol, 1 equiv) and TsOH•H20 (77.9 g, 409 mmol, 3.6 equiv) in MeCN (360 mL) at -5 °C was added NaNO2 (14.12 g, 205 mmol, 1.8 equiv) followed by a solution of CuBr (10.4 mL, 341 mmol, 3 equiv) in H2O (48 mL). The reaction mixture was warmed to room temperature. After 12 h sat. aq. Na2SO3 (200 mL) was added. After 30 min of stirring the reaction mixture was concentrated under reduced pressure to remove organic solvents. The aqueous phase was extracted into EtOAc (3 x 80 mL), then the combined organic phase was washed with sat. aq. NaCl (80 mL), dried over Na2SO4, filtered, and concentrated under reduced pressure. The crude residue was purified by silica gel column chromatography (3→5% EtOAc/petroleum ether) to afford 1-bromo-8-chloronaphthalene (17.2 g, 63% yield) as white solid. 1H NMR (400 MHz, CDCh) δ 7.93 (dd, J = 7.46, 1.22 Hz, 1H), 7.80 (ddd, J= 12.35, 8.19, 0.98 Hz, 2H), 7.67 (dd, J= 7.52, 1.28 Hz, 1H), 7.38 (t, J= 7.83 Hz, 1 H), 7.25-7.32 (m, 1H) [2].
Application
1-Bromo-8-Chloronaphthalene is primarily used as an intermediate in the preparation of a series of quinazoline compounds, and biologicalinhibitors and drug compositions of KRas G12C and KRas G12D that regulate biological processes [3, 4]. These inhibitors have played a significant role in the treatment of central nervous system diseases and other diseases [5].
References
[1] 4-(3,8-Diazabicyclo[3.2.1]octan-3-yl)pyrido[4,3-d]pyrimidines as KRas G12D inhibitors and their preparation. Wang, Xiaolun; Burns, Aaron Craig; Christensen, James Gail; Ketcham, John Michael; Lawson, John David; Marx, Matthew Arnold; Smith, Christopher Ronald; Allen, Shelley; Blake, James Francis; Chicarelli, Mark Joseph et al. From PCT Int. Appl. (2021), WO 2021041671 A1 Mar 04, 2021.
[2] Preparation of 5,6,7,8-tetrahydropyrido[3,4-d]pyrimidin-4-amine and quinazolin-4-amine derivatives as covalent Ras inhibitors and uses thereof. Pitzen, Jennifer; Aggen, James; Burnett, G. Leslie; Gill, Adrian L.; Semko, Christopher; Edwards, Anne V.; Gliedt, Micah James; Kiss, Gert; Jogalekar, Ashutosh; Knox, John E.; Buckl, Andreas; Koltun, Elena S. Assignee Revolution Medicines, Inc., USA. 2021.
[3] Quinazoline derivatives as KRAS G12D inhibitors and their preparation. Han, Huifeng; Gao, Panliang; Zhang, Wenlong; Ma, Cunbo; Wang, Peng; Liu, Dan; Zhang, Hao; Long, Wei Assignee: Jacobio Pharmaceuticals Co., Ltd., Peop. Rep. China.
[4] Preparation of substituted pyrido[4,3-d]pyrimidin-4-amines as KRas G12D inhibitors. Wang, Xiaolun; Lawson, John David; Marx, Matthew Arnold; Allen, Shelley; Barbour, Patrick Michael; Blake, James Francis; Dahlke, Joshua Ryan; Dai, Donghua; Fell, Jay Bradford; Fischer, John Peter; et al. From PCT Int. Appl. (2022).
[5] Quinazoline compounds, preparation methods and uses thereof as inhibitors of KRAS G12D. Dai, Xing; Wang, Yaolin; Jiang, Yueheng; Niu, Haotao; Liu, Yanqin; Yang, Hong; Han, Zixing; Wang, Zhenwu; Tao, Liangshan; Zhang, Qiang; et al. From PCT Int. Appl. (2022).
See also
Lastest Price from 1-Bromo-8-chloronaphthalene manufacturers

US $2.00-5.00/kg2025-06-23
- CAS:
- 20816-79-9
- Min. Order:
- 1kg
- Purity:
- 99%
- Supply Ability:
- 100kg
US $0.00/KG2025-03-21
- CAS:
- 20816-79-9
- Min. Order:
- 1KG
- Purity:
- 98%
- Supply Ability:
- 150KG /month