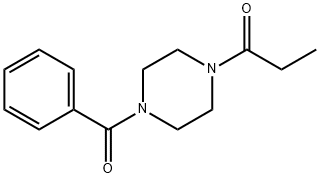
Sunifiram: A Potent Nootropic Agent Targeting Cognitive Enhancement
Sunifiram, also known as DM-235, is an exciting recent development in nootropics. It’s structurally similar to racetam nootropics but about 1,000 times stronger than piracetam. Research suggests that sunifiram may boost brainpower, mood, and energy even when taken in low doses. Sunifiram was first synthesized in 2000 by scientists at the University of Firenze in Italy. Very little research is currently available, but preliminary animal studies indicate that it may be a highly effective nootropic and a potential treatment for various neurological disorders, including Alzheimer’s disease, Parkinson’s disease, and amnesia. No toxicity threshold or serious side effects have been identified, but there have been no documented studies or clinical trials involving humans to date. Animal studies indicated no toxicity, even at high doses. Sunifiram is currently considered an experimental drug, which means it has not been approved for any use in human or veterinary medicine. It is unregulated in the US, where it can be legally purchased, possessed, and used.

Novel Sunifiram-carbamate hybrids as potential dual acetylcholinesterase inhibitor
Nowadays, growing numbers of people complain of cognition impairment (CI) arises through degenerative brain disease like Alzheimer (AD) and Parkinsonism. Individuals with CI are usually in need for expensive nursing, safekeeping, and institutional care.1 Various neurotransmitters are known by their ability to modulate cognitive function; thus they represent potential targets for enhancing cognition. Among these neurotransmitters, acetylcholine (ACh) is well known for its central role in critical physiological processes, such as attention, learning, memory, stress response, wakefulness, sleep, and sensory information. A number of potential strategies for enhancing NMDAR function and hence improving cognition via the glycine site had developed like administration of glycine but this strategy is limited by the high activity of GlyT-1, so effort is moved to develop GlyT-1 inhibitors like Pfizer sarcosine analogue CP-802079. Another promising approach involves exogenous administration of partial agonists like Sunifiram. Sunifiram (DM235) is a novel potent nootropic drug developed by Gualtieri research group in 2000 and is considered as new class of nootropic agents. Sunifiram and related compound Unifram are able to enhance cognitive function four-fold greater than Piracetam in behavioural experiments such as Morris water maze task. These drugs can be helpful in treatment of neurodegenerative disorder like Alzheimer’s, Parkinson’s, multiple sclerosis, schizophrenia, and attention-deficit hyperactivity disorders.[1]
This top compound exhibited relatively similar binding affinity with Sunifira. These docking score hint that this compound favourably bind to the glycine binding pocket. After evaluating the differential docking affinities of the compounds, we proceeded to determine the pharmacokinetics and physicochemical properties of the compounds. In conclusion, novel Sunifiram-carbamate and Sunifram-anthranilamide hybrids were designed, synthesised, and evaluated for cholinergic activity. Introducing carbamate to the skeleton of synthesised targets enabled a good AChE inhibitory activity. Amongst all targets compound the Sunifram-carbamate hybrid 3f showed the most potent AChE inhibitory activity with an IC50 value of 18 ± 0.2 nM. Such ability of 3f together with its good logP value, its ability to induce ACh release from A549 cells, its in vivo ability to lower AChE activity in rat brain makes it worthy of further investigation as a promising nootropic agent. It also showed molecular docking score = −1.7 kcal/mol when docked to glycine binding pocket of NMDA receptor compared to Sunifiram and the anthranilamide hybrid 3i . Compounds 3i further bound preferentially to NMDA domain with high binding affinity interaction which enhanced its binding pocket stability and are promising leads as potent co-agonists that binds to the glycine binding pocket of NMDA receptor and expressed good AChE inhibitory activity with IC50 value of 10.36 ± 0.23 µM.
Novel nootropic drug sunifiram improves cognitive deficits
A progressive neurodegenerative disorder shows cognitive impairment such as Alzheimer's disease (AD). AD is associated with down-regulation of the cholinergic system in the brain. Reduction of glutamatergic system is also found in AD patients. We previously reported that OBX mice show an impaired hippocampal NMDAR function assessed by hippocampal long-term potentiation (LTP). Sunifiram (DM235) is a novel pyrrolidone derivatives, called “nootropics”. Nootropics can be used in several kinds of cognitive deficits of clinical diseases such as AD, Parkinson's disease, multiple sclerosis, schizophrenia and attention-deficit hyperactivity disorders. The mechanism of action of “nootropics” was thought to modulate receptor functions such as the cholinergic system or glutamatergic system. Indeed, sunifiram improves impaired cognitive function induced by scopolamine-induced amnesia when assessed by passive avoidance task or Morris water maze task. The amelioration of acquisition of memory by sunifiram was partly mediated through activation of α-amino-3-hydroxy-5-methyl-4-isoxazolepropionate type glutamate recept. In this present study, we found that the nootropic drug ameliorates deficits of cognitive function by behavioral analyses in vivo. Amelioration of cognitive deficits mediated by sunifiram was associated with stimulation of glycine-binding site of NMDAR.[2]
OBX mice showed both depression-like behaviors and cognitive deficits in our previously reports. Depression-like behaviors in OBX mice are associated with reduced neurogenesis and down-regulation of ERK and Akt activities in the dentate dyrus (DG) of hippocampus. By contrast, cognitive deficits in OBX mice are associated with down-regulation of CaMKII, PKCα and ERK activities and impaired LTP in the CA1 region of hippocampus. In the present study, we examined the biochemical basis for sunifiram effects on improvement of memory and hippocampal LTP and depressive-like behaviors. We also observed that enhancement of LTP by sunifiram concomitant with increased CaMKII autophosphorylation in OBX mouse hippocampus is totally blocked by gavestinel treatment, suggesting that sunifiram preferentially activates CaMKII post-synaptically via stimulation of glycine-binding site of NMDAR. We found that increased autophosphorylation of CaMKII and PKCα through glycine-binding site stimulation was associated with the sunifiram effects in OBX mice. In conclusion, the present study demonstrated that it ameliorates cognitive deficits in OBX mice and it enhances LTP induction via a glycine-binding site of NMDAR activity. The enhancement of LTP by sunifiram increased CaMKII and PKC activities in the CA1. Stimulation of glycine-binding site of NMDAR is deems to important new targets for AD therapeutics.
Sunifiram as a novel nootropic with potential as a cognitive enhancer
DM235 (sunifiram), a new compound structurally related to piracetam, prevented the amnesia induced by scopolamine (1.5 mg kg–1 i.p.), after intraperitoneal or oral (0.01–0.1 mg kg–1) administration, as shown by a passive avoidance test in mice. The antiamnesic effect of DM235 was comparable to that of well-known nootropic drugs such as piracetam (30–100 mg kg–1 i.p.), aniracetam or rolipram. DM235 also prevented mecamylamine (20 mg kg–1 i.p.)-, baclofen (2 mg kg–1 i.p.)- and clonidine-induced amnesia in the same test. In the Morris water maze test with rats, scopolamine (0.8 mg kg–1 i.p.) inhibited the reduction of escape latency in both acquisition and retention/retraining tests.[3]
Sunifiram (0.1 mg kg–1 i.p.), 20 min before each daily acquisition training, prevented the scopolamine-induced memory impairment. DM235 (1 mg kg–1 i.p.) also reduced the duration of pentobarbitone-induced hypnosis in mice without modifying the induction time of hypnosis. At the highest effective doses, the investigated compound neither impaired motor coordination (rota-rod test), nor modified spontaneous motility and inspection activity (Animex and hole board tests). These results indicate that DM235, a compound structurally related to piracetam, is a novel nootropic endowed with the capability to prevent cognitive deficits at very low doses. Indeed, its potency is about 1,000 times higher than that of the most active piracetam-like compounds.
References
[1]Agha KA, Abo-Dya NE, Issahaku AR, Agoni C, Soliman MES, Abdel-Aal EH, Abdel-Samii ZK, Ibrahim TS. Novel Sunifiram-carbamate hybrids as potential dual acetylcholinesterase inhibitor and NMDAR co-agonist: simulation-guided analogue design and pharmacological screening. J Enzyme Inhib Med Chem. 2022 Dec;37(1):1241-1256. doi: 10.1080/14756366.2022.2068147. PMID: 35484855; PMCID: PMC9067966.
[2]Moriguchi S, Tanaka T, Tagashira H, Narahashi T, Fukunaga K. Novel nootropic drug sunifiram improves cognitive deficits via CaM kinase II and protein kinase C activation in olfactory bulbectomized mice. Behav Brain Res. 2013 Apr 1;242:150-7. doi: 10.1016/j.bbr.2012.12.054. Epub 2013 Jan 4. PMID: 23295391.
[3]Ghelardini C, Galeotti N, Gualtieri F, Romanelli MN, Bucherelli C, Baldi E, Bartolini A. DM235 (sunifiram): a novel nootropic with potential as a cognitive enhancer. Naunyn Schmiedebergs Arch Pharmacol. 2002 Jun;365(6):419-26. doi: 10.1007/s00210-002-0577-3. Epub 2002 May 15. PMID: 12070754.
Lastest Price from Sunifiram manufacturers
US $3.00-9.00/KG2025-06-21
- CAS:
- 314728-85-3
- Min. Order:
- 0.1KG
- Purity:
- 99%
- Supply Ability:
- g-kg-tons, free sample is available

US $0.00/kg2025-04-21
- CAS:
- 314728-85-3
- Min. Order:
- 1kg
- Purity:
- 0.99
- Supply Ability:
- 1000kg