Succinic Acid: Solubility and Mechanism of Action
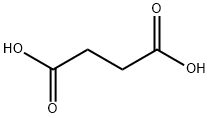
Succinic acid is a dicarboxylic acid that occurs naturally in plant and animal tissues, with the chemical formula (CH2)2(CO2H)2. The name derives from a Latin succinum, meaning amber.
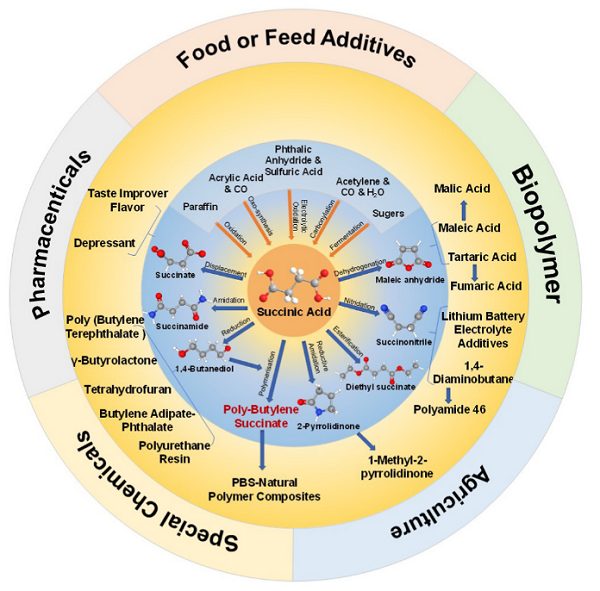
Succinic acid is a common organic acid, which can be used in various food, chemicals, and pharmaceutical industries as a precursor to generate numerous chemicals such as solvents, perfumes, lacquers, plasticizer, dyes, and photographic chemicals. Succinic acid is also used as an antibiotic and curative agent.
Solubility
Succinic acid is soluble in ethanol, ethyl ether, acetone and methanol. Insoluble in toluene, benzene, carbon disulfide, carbon tetrachloride and petroleum ether.
Mechanism of Action
Succinate is an essential component of the Krebs or citric acid cycle and serves as an electron donor in the production of fumaric acid and FADH2. It also has been shown to be a useful "natural" antibiotic because of its relative acidic or caustic nature (extreme concentrations can even cause burns). Succinate supplements have been shown to help reduce the effects of hangovers by activating the degradation of acetaldehyde - a toxic byproduct of alcohol metabolism - into CO2 and H2O through aerobic metabolism. Succinic acid has been shown to stimulate neural system recovery and bolster the immune system. Claims have also been made that it boosts awareness, concentration and reflexes.
You may like
Related articles And Qustion
Lastest Price from Succinic acid manufacturers

US $50.00-10.00/kg2025-07-17
- CAS:
- 110-15-6
- Min. Order:
- 1kg
- Purity:
- 99%,Electronic grade(Single metal impurity≤ 100ppb)
- Supply Ability:
- 200kg

US $0.00/kg2025-05-26
- CAS:
- 110-15-6
- Min. Order:
- 25kg
- Purity:
- 99%min
- Supply Ability:
- 1000kg