Squaric acid analogues in medicinal chemistry
Physicochemical properties
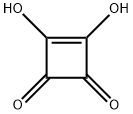
Squaric acid is a symmetrical planar diprotic four-membered oxocarbon compound that possesses unique 2π-pseudo-aromaticity. Consequently, squaric acid features unusual high double acidity (pKa1 = 0.54; pKa2 = 3.58) due to the resonance stabilized squarate dianion (Fig. 1). Several physicochemical methods were employed to confirm the aromatic character of the squarate dianion by geometric (bond length, bond order), energetic (aromatic stabilization energies) and magnetic parameters (17O-chemical shifts, nucleus-independent chemical shifts).

Among the most common squaric acid analogues (Fig. 2), squaramides 5 have received considerable attention especially because of their interesting molecular structure capable of multiple interactions with biological targets via specific molecular recognition. Squaramides can bind selectively through the four hydrogen bonds, acting both as hydrogen bond donors and acceptors. The potential of secondary squaramides to participate in hydrogen bonding is another significant feature which is one of the reasons for their high melting points of 275-300 °C and their low solubility in water. Studies of squaramidebased artificial receptors used for the recognition of cations described that the capability to serve as a hydrogen bond acceptor is modulated by the increase in aromaticity. The ability of squaramides to act as hydrogen bond donors to anionic species was investigated as well. Squaramide-based artificial receptors designed to bind carboxylate anions proved hydrogen bond donor ability also due to enhanced aromaticity. Furthermore, electrostatic potentials suggest that the combined effect of two carbonyls enables squaramides to form strong acceptor interactions more efficiently in comparison with ureas.
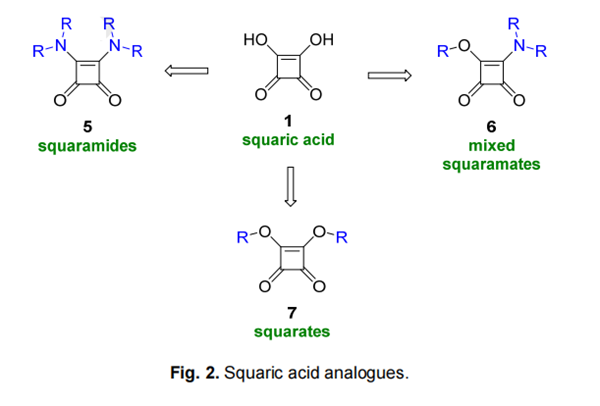
Despite certain similarities between amides and squaramides, the rigid and planar structure of squaramides containing two coplanar carbonyls makes them a distinctive feature. sp2 -Hybridised nitrogens make this arrangement stable by providing their lone pairs to conjugation with the π-system orthogonal to the plane. The mutual influence of a NH and carbonyl oxygen contributes to the formation of zwitterionic structures. Moreover, the dipole presented by a zwitterionic squaramide form is known to mimic the α-ammonium carboxylate motif and thus represents α-amino acid bioisosteres. Amide-like restricted rotation around the C-N bonds forces a bis-secondary squaramide having two C-N bonds to prefer anti/anti conformation.
Squaric acid analogues in medicinal chemistry
Squaric acid analogues are significant in medicinal chemistry for many reasons. First, unlike many other compounds designed as potential drugs, they usually do not suffer from high lipophilicity and low solubility in water. Especially when squaric acid or the squaramide scaffold is combined with amine and carboxylic groups, the resulting compounds show increased solubility, and this makes them suitable therapeutic agents. In addition, squaric acid analogues may serve as non-classical isosteres for carboxylates and amino acids during drug development. For example, N-(hydroxydioxocyclobutenyl)-containing analogues of gamma-amino-butyric acid and L-glutamate were successfully used as analogues of compounds that are active towards amino acid receptors (AMPA and NMDA) in neurons
Mono-amides of squaric acid (squaramates) are often used for bioconjugation with Lys and other amines. Diamides (squaramides) have been used as a phosphate group surrogate in nucleotide or oligonucleotide (ON) analogues. A chemically synthesized 2'-sugar-linked squaramate–RNA conjugate, prepared by the reaction of 2'- amino-modified RNA with diethyl squarate, was reported to cross-link to aminoacyltransferase FemXWv, and this is the only example of the use of a squaric acid analogue in nucleic acid conjugation.
The use of squaric acid analogues in chemical biology is mostly represented by their bioconjugation to proteins or carbohydrates and by their use as ion receptors. In contrast, in medicinal chemistry, these compounds have numerous biological effects, including antiplasmodial, antichagasic, anticancer, and antibacterial activity, which makes them promising agents in drug development.
Conclusions
Squaric acid derivatives, squaramides, squaramines, and squarates are very simple, yet highly useful molecules. Despite these structures being discovered many decades ago by synthetic organic chemists, their use as potential therapeutics has only been explored quite recently. The main reason for this was their appearance; the structures include highly reactive functionalities that are usually associated with negative side-effects, such as toxicity, lack of selectivity, and promiscuity towards various molecular targets.
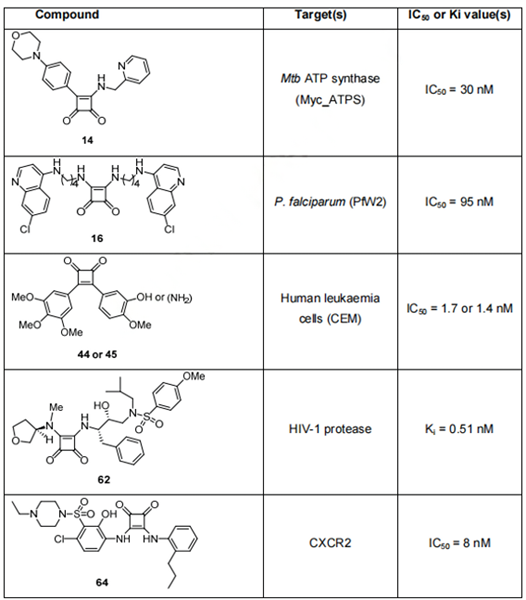
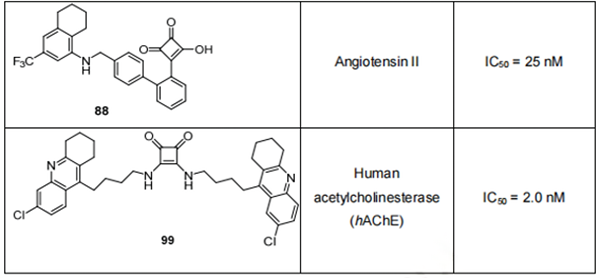
However, such a strict selection of compounds entering the primary screening along with growing drug resistance emerging for the majority of infectious agents, cancers, etc. has resulted in a lack of effective drugs for some of the most prevalent illnesses. This environment has led to renewed attention to some of the previously overlooked compounds, including derivatives of squaric acid. These compounds have a variety of biological activities in low concentrations (often submicromolar), such as antiparasitic, antibacterial, cytotoxic, and antiviral activity, and some have known mechanisms of action (they inhibit certain receptors, enzymes, etc.), which makes them potentially useful in drug development. Ongoing research covering diverse fields of medicinal chemistry has revealed several small squaric acid analogues with significant biological activity; the most potent scaffolds from each group are summarized in Table 1.
References:
[1] JAN CHASÁK . Squaric acid analogues in medicinal chemistry[J]. European Journal of Medicinal Chemistry, 2021. DOI:10.1016/j.ejmech.2020.112872.
You may like
Lastest Price from Squaric acid manufacturers

US $0.00/KG2025-04-15
- CAS:
- 2892-51-5
- Min. Order:
- 1KG
- Purity:
- 99%
- Supply Ability:
- 500000kg

US $80.00/kg2025-04-15
- CAS:
- 2892-51-5
- Min. Order:
- 1kg
- Purity:
- 99
- Supply Ability:
- 5000