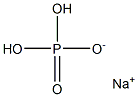
Sodium Dihydrogenphosphate: An Essential Chemical in Various Applications
Introduction
Phosphate is a commonly used reagent for phosphorylation of polysaccharides because it is cheap and easy to obtain. There are mainly disodium hydrogen phosphate, sodium dihydrogenphosphate, sodium trimetaphosphate (STMP), sodium tripolyphosphate (STPP) or their mixed salt[1].
Used to prepare phosphorylated polysaccharide
It has been reported that the phosphorylated polysaccharide can be obtained by mixing STMP and STPP, dissolving them in a particular proportion, adjusting pH, heating reaction, then alcohol precipitation, centrifugation, dialysis and freeze-drying. Feng Xiaoling et al. took split-fold polysaccharide as raw material, added 2 times of STMP-STPP phosphorylation reagent, and reacted at 60 ℃ for 3 h; the degree of substitution of phosphorylated polysaccharide was 0.052. Nanfang Jiang et al. made Ulva lactuca polysaccharide reacted with STMP-STPP phosphorylation reagent (STPP: STMP = 6:1), the phosphorus content of Ulva lactuca polysaccharide phosphate reached 10.47%, and its antioxidant and hypolipidemic activities were improved. Sodium dihydrogenphosphate and disodium hydrogen phosphate are also commonly used. Zhang et al. mixed NaH2PO4 and Na2HPO4 in proportion and then reacted with Bletilla striata polysaccharide with urea as a catalyst. The degree of substitution of phosphate is 0.0138.
Used as a noodle quality improver

Yellow alkaline noodles were prevalent due to the unique alkaline flavour, bright yellow colour, and chewy texture imparted by the addition of alkaline salts, such as sodium or potassium carbonate. However, using alkaline salt in noodles processing could lead to increased susceptibility to browning, which could exacerbate overall browning and lead to the formation of dark spots on the noodles. The appearance of dark colors and spots in the noodles could significantly impact their marketability[2].
Phosphate could be used as a noodle quality improver, and ascorbic acid was commonly used as an anti-browning agent. However, their combined effect on noodle browning has not been extensively investigated. Zhu et al. investigated the impacts and mechanisms of ascorbic acid (Vc) and sodium dihydrogenphosphate on the browning of yellow alkaline noodles. The combination of sodium dihydrogen phosphate and Vc was found to increase the L* value and decrease the number of dark spots, indicating a synergistic anti-browning effect. This combination reduced the activity of polyphenol oxidase (PPO) slightly but decreased the free quinone content significantly (P < 0.05) in noodles. The addition of sodium dihydrogen phosphate also maintained the anti-oxidation stability of Vc, as evidenced by the increased DPPH radical clearance rate in PPO-catechol systems after 24 h of storage. Quantum chemical calculations revealed that sodium dihydrogen phosphate formed hydrogen bonds with ascorbic acid at hydroxyls of carbon-carbon double bond, which protected the ascorbic acid to some degree. This finding suggested that the synergistic anti-browning capacity of sodium dihydrogen phosphate and Vc could be applied to the noodle processing industry.
![Article illustration]() References
References
[1] Sodium Dihydrogen Phosphate - an overview | ScienceDirect Topics https://www.sciencedirect.com/topics/pharmacology-toxicology-and-pharmaceutical-science/sodium-dihydrogen-phosphate
[2] Ying-Ying Tang, Ke-Xue Zhu, Xiao-Na Guo . “Inhibitory mechanism of sodium dihydrogen phosphate and ascorbic acid on browning in yellow alkaline noodles.” Journal of Cereal Science 112 (2023): Article 103706.
Related articles And Qustion
See also
Lastest Price from Sodium phosphate monobasic manufacturers

US $1000.00-920.00/ton2025-07-01
- CAS:
- 7558-80-7
- Min. Order:
- 0.1ton
- Purity:
- 99.0%
- Supply Ability:
- 500

US $0.00/KG2025-04-21
- CAS:
- 7558-80-7
- Min. Order:
- 25KG
- Purity:
- 98%min
- Supply Ability:
- 30tons/month