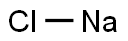Sodium chloride: Introduction; Application and Toxicity
Introduction of Sodium chloride
Sodium chloride, also known as salt, common salt, table salt, or sea salt, is an ionic compound with the chemical formula NaCl. widely distributed throughout the earth, seawater, etc., it is an essential ingredient of the human body, and therefore of the diet. NaCl is also the main salt in seawater and in the extracellular fluid of many multicellular organisms. It is one of the most abundant minerals on earth and an essential nutrient for many plants and animals, including humans. It accounts for more than 90 per cent of the inorganic component of blood serum and is the major salt in maintaining osmotic tension in blood and tissues. It acts as an emetic and flame retardant.

Application of Sodium chloride
Sodium chloride has a wide range of uses in medicine, chemicals, cosmetics and food. It is an essential nutrient and used in healthcare to prevent dehydration in patients; to clean wounds; to clear nasal congestion and keep nasal passages moist; and to treat red, watery and dry eyes. It is available as a food preservative and flavouring, using to add flavour. Sodium chloride is also used as a raw material and intermediate ingredient in synthetic chemicals for the manufacture of other products such as plastics. It is also used to de-ice roads and pavements. It is used in cosmetics for swelling and viscosity control.
Toxicity of Sodium chloride
Too much or too little salt in the diet can lead to muscle cramps, dizziness or electrolyte disturbances that can lead to neurological problems or death. Ingestion of large amounts of salt (about 1 gram per kilogram of body weight) in a short period of time can lead to death. Deaths have also resulted from attempts to use salt solutions as an emetic, forced salt intake, and accidental confusion of salt with sugar in children's food. Chronic or prolonged excessive salt intake can lead to stroke, high blood pressure, left ventricular hypertrophy and stomach cancer.
A diet high in salt disrupts the body's natural sodium balance. This can lead to fluid retention, which increases the pressure exerted by the blood on the walls of blood vessels, resulting in hypertension or high blood pressure. It is estimated that reducing salt intake from 10 grams to 6 grams per day could lower blood pressure sufficiently to reduce deaths from stroke by 16 per cent and deaths from coronary heart disease by 12 per cent.
References:
[1] KOTCHEN T A. Contributions of sodium and chloride to NaCl-induced hypertension.[J]. Hypertension, 2005, 45 5: 849-850. DOI:10.1161/01.HYP.0000164629.94634.27.
Related articles And Qustion
Lastest Price from Sodium chloride manufacturers

US $0.00-0.00/kg2025-12-13
- CAS:
- 7647-14-5
- Min. Order:
- 1kg
- Purity:
- 99.9%
- Supply Ability:
- 1000kg

US $10.00/KG2025-04-21
- CAS:
- 7647-14-5
- Min. Order:
- 100KG
- Purity:
- 99%
- Supply Ability:
- 100 mt