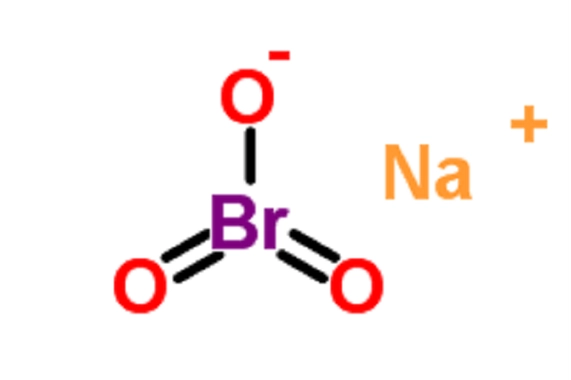
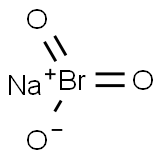Sodium bromate: Production, Uses and Toxicity
Production and Uses of Sodium bromate
Sodium bromate is produced by passing bromine through a solution of sodium carbonate. Sodium bromate is used as an analytical reagent, for the oxidation of sulphur and reducing dyes, and for cleaning boilers. As a mixture with sodium bromide, it is used to dissolve gold from ores. Sodium bromate and potassium bromate are used by the cosmetic industry as neutralising or oxidising agents in hair-wave preparations. In 1991, the cosmetic industry voluntarily reported to the United States Food and Drug Administration (FDA) the presence of sodium bromate in 61 cosmetic formulations, in concentrations ranging from 10% to 25%.
Toxicity of Sodium bromate
Several cases of acute bromate intoxication have been reported in humans following accidental or suicidal ingestion of permanent hair wave neutralizing solution. These products usually contain either 2% potassium bromate or 10% sodium bromate. Bromate intoxication leads to gastrointestinal symptoms (abdominal pain, nausea, vomiting, diarrhea), central nervous system depression, renal failure, and hearing loss. Bromate intoxication has been reported to be lethal.
Kidney effects are frequently observed in children and adults following acute exposure with sodium and potassium bromate and include anuria, oliguria, and renal failure. A review of bromate kidney toxicity found that renal failure occurred in 26 of 31 reported cases. Histological examination of renal biopsies from children with renal effects indicated interstitial edema, tubular atrophy and dilation, interstitial fibrosis, and epithelial separation of the proximal tubules
Bromate exposure produces irreversible deafness in children and adults. Quick et al. (1975) described a 6-year-old boy who ingested approximately 0.5 grams of 95% pure potassium bromate pellets. The serum bromide level of this patient was 17 mg/100 mL. described a 17-month-old girl who ingested an unknown amount of permanent wave neutralizer solution containing potassium bromate. Both children developed vomiting, diarrhea, and anuria. Audiographic examinations indicated that the children had severe bilateral hearing loss. Matsumoto et al. (1980) reported two cases of women who ingested 10 and 15 grams of potassium bromate, respectively, and lost their hearing within 12 hours of ingestion. A review of bromate ototoxicity by these authors found that deafness occurred in 17 of 20 cases in Japan usually 4 to 16 hours after ingestion.
References:
[1] Toxicology studies of sodium bromate (CAS No. 7789-38-0) in genetically modified (FVB Tg.AC Hemizygous) mice (dermal and drinking water studies) and carcinogenicity studies of sodium bromate in genetically modified [B6.129-Trp53tm1Brd (N5) haploinsufficient] mice (drinking water studies).[J]. National Toxicology Program genetically modified model report, 2007, 6: 1-169.
You may like
See also
Lastest Price from Sodium bromate manufacturers

US $10.00/KG2025-04-21
- CAS:
- 7789-38-0
- Min. Order:
- 1KG
- Purity:
- 99%
- Supply Ability:
- 10 mt

US $20.00-1.00/kg2025-03-07
- CAS:
- 7789-38-0
- Min. Order:
- 1kg
- Purity:
- 0.99
- Supply Ability:
- 10 tons