Rivaroxaban
Introduction
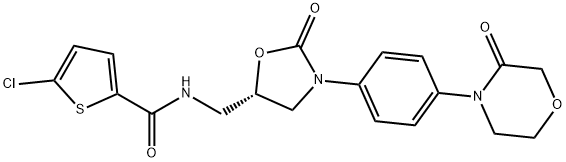
Rivaroxaban (Xarelto®), an oral oxazolidinone-based anticoagulant, is a potent, selective direct inhibitor of factor Xa that is used in the prevention of venous thromboembolism (VTE) in adult patients after total hip replacement (THR) or total knee replacement (TKR) surgery. In four large, clinical trials, oral rivaroxaban was more effective than subcutaneous enoxaparin in preventing postoperative VTE in patients undergoing THR or TKR surgery. Notably, the superior efficacy of rivaroxaban was achieved with a low but not significant increase in the incidence of major bleeding episodes. In addition, preliminary pharmacoeconomic analyses in several countries indicate that rivaroxaban is a cost-effective treatment strategy versus enoxaparin. Although the position of rivaroxaban relative to other therapies remains to be fully determined, it is an effective emerging option for the prevention of VTE following THR and TKR.
Pharmacological properties
Rivaroxaban is a potent oral direct inhibitor of the serine endopeptidase factor Xa and inhibits both free factor Xa and factor Xa bound in the prothrombinase complex. The potency of factor Xa inhibition occurs primarily as a result of rivaroxaban binding with high selectivity to the S1 and S4 pockets of the serine endopeptidase.
In healthy volunteers and in orthopaedic surgery patients, rivaroxaban prophylaxis dose-dependently inhibited factor Xa and prolonged prothrombin (PT) and activated partial thromboplastin times confirming data from animal models. Plasma rivaroxaban concentrations correlated with both factor Xa inhibition and PT, following a maximum effect and linear model, respectively. Rivaroxaban prophylaxis did not prolong the corrected QT interval using the Fridericia formula.
Rivaroxaban pharmacokinetics following oral administration are best described by a one-compartment model and are characterized by rapid absorption (peak plasma concentration reached within 2–4 hours) and high bioavailability (80–100%). The apparent volume of distribution of rivaroxaban at steady state is ≈50 L. Rivaroxaban can be classified as a low-clearance drug, with a mean apparent oral clearance rate in orthopaedic surgery patients of 5.5 L/h on the day of surgery, increasing to 7.5 L/h at steady state, and mean terminal half-life of between 7 and 11 hours.
Approximately two-thirds of an administered rivaroxaban dose is metabolized via cytochrome P450 (CYP) enzymes (CYP3A4 and CYP2J2) and CYP-independent mechanisms, with one-third excreted as unchanged drug in the urine. The metabolized fraction of the drug is eliminated in approximately equal amounts via the renal and faecal routes.
The efficacy and safety of oral rivaroxaban
Rivaroxaban is an oral, direct Factor Xa inhibitor, which is at an advanced stage of clinical development for prevention and treatment of thromboembolic disorders. Two phase II studies, ODIXa-DVT and EINSTEIN DVT, assessed the efficacy and safety of oral rivaroxaban (once daily or twice daily) for treatment of acute deep-vein thrombosis (DVT). Population pharmacokinetic and pharmacodynamic analyses of rivaroxaban in patients in these two phase II studies were conducted to characterize the pharmacokinetics/pharmacodynamics of rivaroxaban and the relationship between important patient covariates and model parameters. Exposure simulations in patients with atrial fibrillation (AF) were also performed in order to predict the exposure of rivaroxaban, using modified demographic data reflecting the characteristics of a typical AF population.
The pharmacokinetics of rivaroxaban in patients with DVT were found to be consistent and predictable across all doses studied. The area under the plasma concentration-time curve (AUC) increased dose dependently. The same total daily doses given once daily achieved higher maximum plasma concentration (Cmax) values (∼20%) and lower trough (minimum) plasma concentration (Ctrough) values (∼60%) than when given twice daily; however, the 5th–95th percentile ranges for these parameters overlapped. Rivaroxaban clearance was moderately influenced by age and renal function, and the volume of distribution was influenced by age, body weight and sex; the effects were within the observed interindividual variability. Simulations in virtual patient populations with AF showed that a rivaroxaban dose of 15 mg once daily in patients with creatinine clearance of 30–49 mL/min would achieve AUC and Cmax values similar to those observed with 20 mg once daily in patients with normal renal function. The prothrombin time correlated almost linearly with plasma rivaroxaban concentrations (≤500 µg/L).
Reference
1 Duggan S T, Scott L J, Plosker G L. Rivaroxaban[J]. Drugs, 2009, 69(13): 1829-1851.
2 Mueck W, Lensing A W A, Agnelli G, et al. Rivaroxaban[J]. Clinical pharmacokinetics, 2011, 50(10): 675-686.
Related articles And Qustion
See also
Lastest Price from Rivaroxaban manufacturers

US $0.00/kg2025-06-30
- CAS:
- 366789-02-8
- Min. Order:
- 1kg
- Purity:
- 98%
- Supply Ability:
- 1000kg

US $5.00-0.50/KG2025-05-16
- CAS:
- 366789-02-8
- Min. Order:
- 0.10000000149011612KG
- Purity:
- 99% hplc
- Supply Ability:
- 5000kg