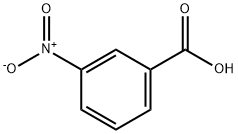
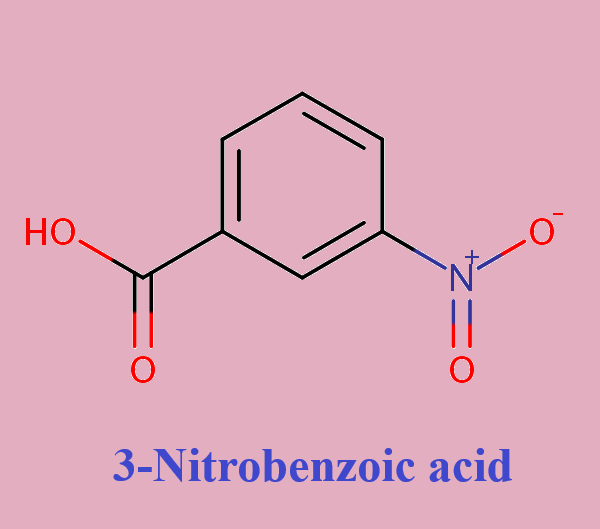
Reactivity and Polarity of 3-Nitrobenzoic acid
3-Nitrobenzoic acid is an off-white solid, it is used to prepare some dyes.
Properties
With a pKa of 3.47,3-nitrobenzoic acid is about ten times more acidic than benzoic acid. The conjugate base of benzoic acid is stabilized by the presence of the electron-withdrawing nitro group which explains its increased acidity in comparison to unsubstituted benzoic acid. It is typically soluble in oxygenated and chlorinated solvents.
Reactivity
The presence of both carboxylic acid and nitro functional groups deactivates the ring concerning electrophilic aromatic substitution reactions.
Polarity
3-Nitrobenzoic acid is a polar compound. This is because of the unbalanced electron density. The electronegativity difference between hydrogen and carbon is negligible, whereas the electronegativity difference between carbon and oxygen, nitrogen and oxygen is larger enough to cause polarity.
You may like
Related articles And Qustion
See also
Lastest Price from 3-Nitrobenzoic acid manufacturers

US $10.00/KG2025-04-21
- CAS:
- 121-92-6
- Min. Order:
- 1KG
- Purity:
- 99%
- Supply Ability:
- 10 mt
US $15.35/Kg/Drum2025-04-21
- CAS:
- 121-92-6
- Min. Order:
- 25Kg/Drum
- Purity:
- 99.50%HPLC
- Supply Ability:
- 10tons/month