Property, preparation and application of lithium hydroxide monohydrate
General description
Name: Lithium hydroxide monohydrate
CAS: 1310-66-3
Molecular formula: LiOH•H2O (H3LiO2)
Molecular weight: 42.0386
Appearance: White crystalline powder
Melting point: 462 °C
Boiling point: 924 °C
Water solubility: 109 g/L (20℃)
Density: 1.46 g/cm3
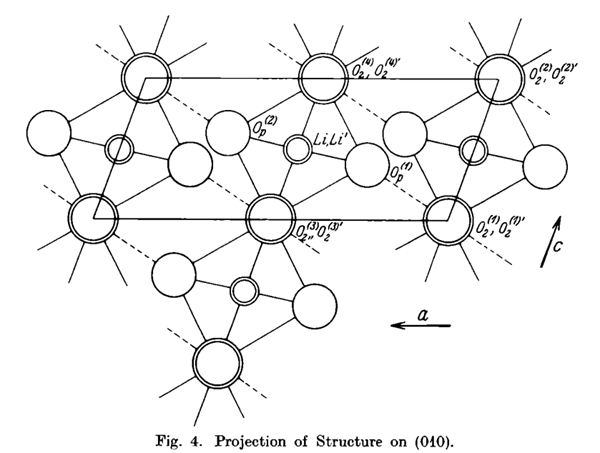
Fig. 1 The structure of lithium hydroxide monohydrate.
Physical and chemical properties
Lithium hydroxide monohydrate is soluble in water, slightly soluble in alcohol, as well as strong alkalinity, no flammability, but high corrosion [1]. It is susceptible to deterioration by absorption of carbon dioxide and water from the air [2].
Preparation process
The main raw materials of monohydrate lithium hydroxide are lepidolite, spodumene, etc. The preparation methods include the following categories.
(1) Limestone roasting
The ore containing lithium is mixed with limestone in a certain mass ratio and ground into fine powder. Then the ground slurry is sent to the rotary kiln for calcination, and the CaO produced by the decomposition of calcium carbonate reacts with lithium ore to form LiOH•H2O. However, due to the disadvantages of high energy consumption, large material flow, high cost and great difficulty to improve product quality, this process has been rarely used [3].
(2) Pressure leaching about β-spodumene and sodium carbonate
The α-spodumene concentrate is roasted in the rotary kiln at 1050 °C-1100 °C until it is transformed into spodumene, and a certain amount of Na2CO3 is added to mix it evenly and then leached at 200 °C. CO2 is added to generate the soluble LiHCO3, and the residue is filtered, and then refined lime milk is added according to stoichiometric ratio, and finally the reaction solution is condensed and crystallized to obtain LiOH•H2O [4].
(3) Causticization of lithium carbonate
First of all, refined lime milk and lithium carbonate are mixed in a certain proportion, and the concentration of causticizing liquid is adjusted, heated to boiling and strongly stirred, and the concentration of about 3.5% LiOH solution is obtained. The insoluble residue (mainly CaCO3) is removed, and the mother liquor is concentrated and crystallized under reduced pressure after heart separation to obtain lithium hydroxide monohydrate. Subsequently, the product is dried at 130 °C and 140 °C, and then reduced pressure and heated at 150 °C and 180 °C to produce anhydrous LiOH. Causticization of lithium carbonate is currently the main way to produce lithium hydroxide at home and abroad. However, the technique is complex and costly. Especially, the main raw material is lithium carbonate, whose price directly affects the cost of lithium hydroxide monohydrate [5].
(4) Electrolytic refined brine
The brine is concentrated to 5%-7% Li. After filtration, the pH is adjusted to 10.5-11.5, and calcium and magnesium ions in the brine are removed by precipitation to obtain the refined brine (mainly LiCl). Then, the refined brine is electrolyzed in a special electrolytic cell as the electrolyte. The anode electrolyte is refined brine, and the cathode electrolyte is water or LiOH solution. There is a cation selective permeable membrane (RF-SO3H, RF-COOH, etc.) between the anode and cathode electrolyte. Cations can pass through the membrane, while anions are not. During electrolysis, Li+ can be transferred to the cathode and converted into LiOH. The pressure and mangles produced by the reaction can be used as by-products to produce HC1. Finally, LiOH solution with a concentration of about 14% can be obtained at the cathode, and LiOH products can be obtained by crystallization and drying. However, this method has high energy consumption, high cost and great impact on environment [6].
(5) Calcination method
Boron is extracted from the brine and 50% of the water was evaporated and calcined at 700 °C. The magnesium chloride in the brine is pyrolyzed to magnesium oxide with a decomposition rate of 93%, and then soaked with water. The leached solution (containing 0.14% lithium) is added with lime milk and soda ash to remove calcium and magnesium ionons, and added with Na3POjX to produce Li3PCV for filtration. Li3PO4 precipitate was mixed with CaO and Al2O3 at a ratio of 162, and then calcined at 2300 °C in a resistance furnace. Then the calcined mixture was leached with hot water at 85 °C and 95 °C, filtered, and the filtrate was evaporated, concentrated, crystallized and dried to obtain LiOH products. The advantages of this method are that lithium and magnesium can be comprehensively utilized and less chemical raw materials are needed. Calcination can remove impurities such as borax and magnesium and improve the purity of lithium hydroxide. The deficiency point is that the use of magnesium makes the process process complex, serious corrosion of equipment, large evaporation water, high energy consumption [7].
(6) Electrolytic Li2SO4 solution
Li2SO4 solution as anode and water as cathode were placed in the membrane electrolytic cell device for electrolysis. The anode electrolyte and cathode electrolyte were separated by fluorinated cation exchange resin (such as heteromolecular polymer of C2H4 and CT2=CFO (CF2) 3 COCFS), and the control voltage was 6 V. The current density is IOOA/dm2. Eventually, LiOH solution with a mass concentration of 10% can be obtained at the cathode, and H2SO4 solution can be obtained at the anode. LiOH prepared by ion membrane electrolysis has high recovery rate of Li (nearly 100%), no secondary pollution, and high purity (> 99%), which can be directly used to produce lithium lubricant. However, this method requires very high content of impurity ions in refined brine. The total concentration of Na+ and K+ should be below 5%, and the total concentration of Ca2+ and Mg2+ should not exceed 0.004%. In addition, the ionic membrane is expensive and difficult to maintain, which increases the production cost of LiOH [8].
Application
Lithium hydroxide monohydrate is one of the most important lithium compounds, which is often used as raw material for lithium compounds, and can also be used in metallurgy, petroleum, glass, ceramics and other industries [9]. Herein, lithium hydroxide is one of the most important lithium salts. In addition, it is also widely used in chemical raw materials, chemical reagents, magnesium ion batteries, petroleum, metallurgy, glass Li, ceramics and other industries. At present, manufacturing advanced lithium grease is the field with the largest consumption of LiOH•H2O. Because the lithium grease produced by LiOH•H2O has wide applicable temperature range (50 °C-300 °C), good fire resistance, difficult oxidation, stable performance during multiple heating cooling heating cycles, long service life and strong water resistance. Moreover, LiOH•H2O plays an important role in the chemical, defense, nuclear, aerospace, and battery industries. It is used in the battery industry as an additive for alkaline batteries to prolong their life and increase charge storage. In the field of national defense, it is not only used as an ion exchange resin to absorb radioactive isotopes, but also as a heat carrier for nuclear reactors and a protective agent for metal surfaces. In aerospace, it can be used for air purification in submarines and pilots' breathing hoods [10]. Besides, LiOH•H2O can be used as raw materials for purifying agent, emulsifier, special optical glass, vitamin A and many other lithium salt products.
Risk and safety
Corrosion resistance.
Harmful by inhalation and if swallowed.
Cause severe burns.
Do not inhale dust.
In case of contact with eyes, rinse immediately with plenty of water and seek medical advice.
Wear appropriate protective clothing, gloves and goggles or masks.
In case of accident or if you feel unwell, seek medical advice immediately (show the label whenever possible).
References
[1] J.C. Kelly, M. Wang, Q. Dai, O. Winjobi, Energy, greenhouse gas, and water life cycle analysis of lithium carbonate and lithium hydroxide monohydrate from brine and ore resources and their use in lithium ion battery cathodes and lithium ion batteries, Resources, Conservation and Recycling 174 (2021) 105762.
[2] G. Gadikota, Connecting the morphological and crystal structural changes during the conversion of lithium hydroxide monohydrate to lithium carbonate using multi-scale X-ray scattering measurements, Minerals 7(9) (2017) 169.
[3] P. Braga, S. França, R. Neumann, M. Rodriguez, G. Rosales, Alkaline process for extracting lithium from Spodumene, Proceedings of the 11th International Seminar on Process Hydrometallurgy-Hydroprocess, Santiago, Chile, 2019, pp. 19-21.
[4] N.K. Salakjani, P. Singh, A.N. Nikoloski, Production of lithium–A literature review. Part 2. Extraction from spodumene, Mineral Processing and Extractive Metallurgy Review 42(4) (2021) 268-283.
[5] H. Liu, G. Azimi, Production of Battery Grade Lithium Hydroxide Monohydrate Using Barium Hydroxide Causticizing Agent, Resources, Conservation and Recycling 179 (2022) 106115.
[6] J.C. Kelly, M. Wang, Q. Dai, O. Winjobi, Energy, greenhouse gas, and water life cycle analysis of lithium carbonate and lithium hydroxide monohydrate from brine and ore resources and their use in lithium ion battery cathodes and lithium ion batteries, Resources, Conservation and Recycling 174 (2021) 105762.
[7] Y. Lee, J. Lee, K.Y. Lee, J. Mun, J.K. Lee, W. Choi, Facile formation of a Li3PO4 coating layer during the synthesis of a lithium-rich layered oxide for high-capacity lithium-ion batteries, Journal of Power Sources 315 (2016) 284-293.
[8] N. Nemkov, A. Ryabtsev, N. Kotsupalo, L. Menzheres, E. Mamylova, O. Chayukova, Preparing High-Purity Lithium Hydroxide Monohydrate by the Electrochemical Conversion of Highly Soluble Lithium Salts, Theoretical Foundations of Chemical Engineering 54(4) (2020) 710-718.
[9] X. Yang, H. Huang, Z. Wang, M. Kubota, Z. He, N. Kobayashi, Facile synthesis of graphene oxide-modified lithium hydroxide for low-temperature chemical heat storage, Chemical Physics Letters 644 (2016) 31-34.
[10] V. Gorelik, D. Bi, Y. Voinov, A. Vodchits, B. Gorshunov, N. Yurasov, I. Yurasova, Raman spectra of lithium compounds, Journal of Physics: Conference Series, IOP Publishing, 2017, p. 012035.
Related articles And Qustion
See also
Lastest Price from Lithium hydroxide monohydrate manufacturers

US $1.00-4.00/KG2025-05-19
- CAS:
- 1310-66-3
- Min. Order:
- 1KG
- Purity:
- 99%
- Supply Ability:
- 200000KG

US $3.00/kg2025-04-21
- CAS:
- 1310-66-3
- Min. Order:
- 1kg
- Purity:
- 0.99
- Supply Ability:
- 10000