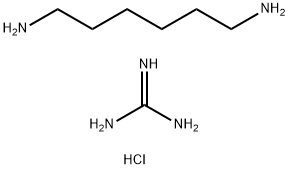
Preparation of polyhexamethylene guanidine hydrochloride
Introduction
Polyhexamethylene guanidine hydrochloride (PHMG) is a new environment-friendly guanidine disinfectant, which has extremely strong bactericidal and bacteriostatic properties, broad spectrum and high efficiency, long validity period, non-toxic side effects, no corrosivity and irritation, safe to use, and can reduce[1]. It is environmentally friendly and pollution-free. Therefore, polyhexamethylene guanidine hydrochloride has been applied well in many fields. Polyhexamethylene guanidine hydrochloride can be used as a disinfectant on the surface of objects, an antiseptic for daily necessities, and a disinfectant for wet wipes; Fungicides for food processing, building materials and oil exploitation; Disinfectants and algaecides for aquaculture, swimming pool, lake water, etc; It can also be used as disinfectant for livestock farms, fabric surface treatment agent, sewage treatment flocculant, polymer antibacterial modifier, etc[2].
Physical and chemical properties
The appearance of polyhexamethylene guanidine hydrochloride is a white powdery solid, which is easily soluble in water and becomes a colorless to yellowish liquid after being dissolved in water; Its aqueous solution is incombustible, non explosive, tasteless, almost non corrosive to most metal materials, safe to use, and its decomposition temperature exceeds 400 ℃, biodegradable in the natural environment, and will not cause secondary pollution to the environment [3].
Preparation of polyhexamethylene guanidine hydrochloride
Since the 1930s, a series of guanidinium salts have been reported to have good bactericidal properties, and guanidinium salt derivatives have been widely used in medical, plant protection, industry, food and other fields as fungicides and antimicrobials. Since the 1990s, polyhexamethylene guanidine salt with high bactericidal effect has been developed and received extensive attention [4]. Polyhexamethylene guanidine hydrochloride is mostly obtained through the condensation reaction of guanidine hydrochloride and hexamethylene diamine. The reaction equation is as follows:
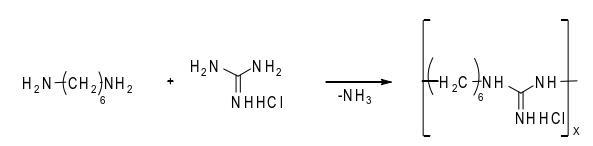
Guanidine hydrochloride is a trifunctional compound and hexamethylene diamine is a bifunctional compound. Theoretically, the polycondensation of the two raw materials can produce a polymer. However, because the=NHHCl functional group in guanidine hydrochloride is an ion pair, its reaction activity is higher than that of the other
The other two amino groups have low functional groups. Therefore, the linear product of polyhexamethylene guanidine hydrochloride [5] can be obtained by controlling the reaction conditions. Only excessive hexamethylene diamine can cross link with the third amino group of guanidine hydrochloride and form a polymer [5]. The cross-linked polymer is insoluble and non fusible, so it can not give full play to the bactericidal effect. Therefore, the preparation of linear, narrow distribution, water-soluble PHMG polymer with a certain molecular weight range is an important condition to ensure its bactericidal performance. Martin Albert et al. [6] studied the effect of molecular weight on the bactericidal properties of polyguanides, and found that the molecular weight range of 800~1300Da has good bactericidal activity.
Starting agent and terminating agent adding method
In order to control the molecular weight and molecular weight distribution of polyhexamethylene guanidine hydrochloride, the method of adding initiator and terminator can be adopted. Chinese patent CN101245141A [6] reported that starting agent and terminating agent can be added during reaction. Add guanidine hydrochloride and triethylenediamine into the reactor at a molar ratio of 1:1, and add a starting agent accounting for 1 ‰ of the total weight of reactants. Gradually raise the temperature and react at 100 ℃, 150 ℃, 200 ℃ and 250 ℃ respectively. Before the reaction is terminated, add a terminating agent accounting for 1 ‰ of the total weight of reactants to obtain the initial polymer melt. Then transfer the initial polymer melt into the hydrolysis tank, add purified water to prepare a 10% solution, and use the ion separation exchange membrane separator to separate the polyhexamethylene guanidine hydrochloride from the solution. The starting agent is choline chloride or azodiisoheptanitrile, and the terminating agent is PTL or butadiene.
Reference
1 Yurevich, V. P.; Efimov, K. M.; Martynenko, S. V. etc. Production of polyguanidine salts by melt polymerization of diamines and guanidine salts Full Text Russ. RU 2318803 C1 20080310.
2 Nyzhnyk, Juriy Vasyliovych; Baranova, Ganna Ivanivna; Marievskyi, Viktor Fedorovych etc. Method for producing polyguanidines with high biological activity for antiseptics and cosmetics. WO 2008039162 A1 20080403.
3 Bardeau, Jean-Francois; Tabellout, Mohamed; Rogalskiy, Sergiy; Tarasyuk, Oksana; Fatyeyeva, Kateryna . Novel antimicrobial composition use and preparation thereof. WO 2011131773 A1 20111027.
4 Yu Feng. Fungicide raw material polyhexamethylene guanidine hydrochloride and its preparation method China: CN102369944 A [P] 2010-08-11.
5 Yu Gang. Polyhexamethylene guanidine hydrochloride and its preparation method China: CN101245141A [P]. 2008-08-20
Related articles And Qustion
See also
Lastest Price from Polyhexamethyleneguanidine hydrochloride manufacturers

US $1.00/kg2025-04-21
- CAS:
- 57028-96-3
- Min. Order:
- 1kg
- Purity:
- 99%
- Supply Ability:
- 10 mt

US $0.00/Kg/Drum2025-04-21
- CAS:
- 57028-96-3
- Min. Order:
- 100g
- Purity:
- ≥99% ≥95% ≥55% ≥25%
- Supply Ability:
- 2000mt