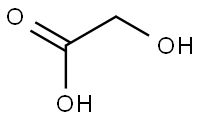
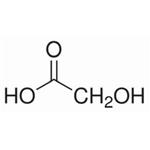
Preparation of Glycolic acid
Glycolic acid is available commercially as either a 57 % (Hoechst) or a 70 % (Du Pont) aqueous solution. Total annual consumption worldwide is ca. 2000 – 3000 t of solution. Glycolic acid is used in textile dyeing, printing, and creaseproofing. The fact that it can form a chelate with calcium(II) ions makes it wellsuited to hide deliming in the leather industry, as well as to inclusion in alum and chrome mordants and in fur-processing operations.
Chemical Properties
The compound has little tendency to cause corrosion, and this characteristic, coupled with its bactericidal properties, makes it suitable for incorporation into acidic cleansing agents. It is especially welladapted to cleansing operations involving milk containers, milk-processing equipment, and drinking fountains, as well as rust and scale removal in heat exchangers and pipelines. Glycolic acid inhibits the growth of iron-oxidizing bacteria. The use of glycolic acid eliminates the need for simultaneous addition of chelating agents and bactericides. The effectiveness of glycolic acid as a complexing agent also contributes to its use in copper polishes, as an etching agent for lithographic plates, and in the preparation of electropolishing and galvanizing baths.
Analysis.
Glycolic acid may be detected qualitatively by the violet color formed with 2,7-dihydroxynaphthalene [17]. The preferred method of quantitative analysis (in the absence of other acidic or hydrolyzable substances) is acidimetric titration. Because of the tendency of lactide formation free and total acid must be determined separately.
Preparation
Glycolic acid is usually produced by hydrolysis of molten monochloroacetic acid with 50 % aqueous sodium hydroxide at 90 – 130℃. The resulting glycolic acid solution has a concentration of ca. 60 % and contains 12 – 14 % sodium chloride. The salt may be removed by evaporative concentration, followed by extraction of the acid with acetone. Attempts have also been made to conduct the hydrolysis with acid catalysts at 150 – 200℃ with water or steam under pressure. In this case, the byproduct is hydrogen chloride, rather than sodium chloride, which can be removed by distillation. The principal disadvantage of the method is the need for relatively large volumes of water.
Glycolic acid is produced commercially in the United States (Du Pont) by treating formaldehyde or trioxymethylene with carbon monoxide and water in the presence of acid catalysts at >30 MPa. Another process, previously utilized by Degussa, involves electrolytic reduction of oxalic acid. The compound can also be prepared in 90 % yield by hydrolysis of the corresponding nitrile, obtained by reaction of formaldehyde with hydrocyanic acid.
You may like
Related articles And Qustion
See also
Lastest Price from Glycolic acid manufacturers
US $3.80-3.00/KG2025-07-18
- CAS:
- 79-14-1
- Min. Order:
- 1000KG
- Purity:
- 70-72%
- Supply Ability:
- 500 tons

US $0.00-0.00/kg2025-06-11
- CAS:
- 79-14-1
- Min. Order:
- 0.001kg
- Purity:
- 99.99%
- Supply Ability:
- 20000T