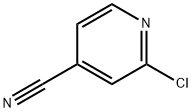
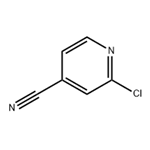
Preparation of 2-Chloro-4-cyanopyridine
2-Chloro-4-cyanopyridine is an important organic intermediate to synthetize substituted pyridines. 2-Chloro-4-cyanopyridine can be prepared according to the reported literatures[1-6].
Method 1

In a 1000ml reaction flask, add 4-cyanopyridine-N-oxide 120g,1,2-dichloroethane 360ml, phosphorus oxychloride 183.6g, cool the reaction system to -2±2°C, triethylamine 151.5g was added dropwise. The dripping time is 2h. After the dripping is completed, the incubation time is 2h. After the reaction is completed, the reaction solution was concentrated until no more fractions flowed out. Add 240 ml water, Stirring. There are a lot of solids out, Suction filtration. The filter cake is rinsed with water. 117.5g of white solid. That is 2-chloro-4-cyanopyridine.
Method 2

POCl3 (9.0L, 97.4 mol) was added to 4-cyanopyridine N-oxide (3.0 kg, 24.98 mol) and the slurry slowly heated to 80° C. After 5h at 80° C, the reaction mixture became a clear solution and was warmed to 100° C. and aged for 24 h. After cooling to RT, the slurry was slowly added to pH 7 buffer (30L). During the addition, the temperature was kept at 15-35° C. and the pH was maintained at approx. 5-6 by addition of NaOH (9.6 N, 21L). The product was extracted into MTBE (18L) and the organic layer was treated with charcoal (Darco G-60, 600 g) for 4 h. The slurry was filtered over celite and washed with MTBE. The solvent was switched from MTBE to heptane and the slurry filtered to recover the product. Analytical data consistent with that reported in the literature: Rokash, J. and Girard, Y. J. Heterocyclic Chem. 1978, 15, 683.
Method 3
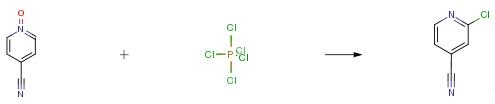
A mixture of 4-cyanopyridine N-oxide (30 g, 0.25 mol), phosphorous oxychloride (96 ml, 0.35 mol) and phosphorous pentachloride (72 g, 0.38 mol) was refluxed at 120°-130° C for 6 hours and then stirred at room temperature for 12 hours. The reaction mixture was slowly poured into a mixture of ice/Na2CO3 /K2CO3 and was extracted with chloroform (4*250 ml). The solvent was removed in vacuo and the residue was purified by column chromatography on silica eluding with 50 percent ether/hexanes to afford 8.4 g (24percent) of 2-chloro-4-cyanopyridine, m.p. 69.5°-70.5° C. and 10.5 g (30percent) of 3-chloro-4-cyanopyridine, m.p. 71.5°-72° C.
References
1. Shandong Jincheng Pharmaceutical And Chemical Co., Ltd. Sun B, Yi M, Zhang N, Ma Q, Wang X. He holds the pyrrole department preparation method (by machine translation). CN104945383[P], 2017, B. Paragraph 0031; 0035; 0036.
2. Marcantonio K, Frey L, Frantz D, Murry JA, Tillyer R. Synthesis of substituted imidazoles. US2003/55255[P], 2003, A1.
3. Sterling Winthrop Inc. 6-heterocyclyl pyrazolo [3,4-d]pyrimidin-4-ones and compositions and method of use thereof . US5294612[P], 1994, A
You may like
See also
Lastest Price from 2-Chloro-4-cyanopyridine manufacturers

US $1.10/g2025-04-17
- CAS:
- 33252-30-1
- Min. Order:
- 1g
- Purity:
- 99.0% min
- Supply Ability:
- 100 tons min
US $0.00-0.00/KG2025-04-04
- CAS:
- 33252-30-1
- Min. Order:
- 1KG
- Purity:
- 98%
- Supply Ability:
- 1ton