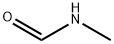Preparation and Application of N-Methylformamide
General description
N-Methylformamide is an important organic synthetic raw material, used in the synthesis of pesticide insecticide and acaricide monamidine, dimethylamidine, also used in the production of medicine, synthetic leather, artificial leather and as a chemical fiber textile solvent.
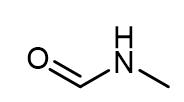
Fig. 1 The structure of N-Methylformamide.
Physicochemical property
The pure product of N-methylformamide is a colorless transparent viscous liquid with a melting point of.3.8 °C and a boiling point of 198-199 °C. Its density at 25 °C is 1.011 g/mL. It is soluble in water, can also dissolve inorganic salts, is hygroscopic, in acidic or alkaline solutions easily decomposed.
Preparation
1. Methylamine method
CO+CH3NH2→ HCONHCH3
69.62% (mass fraction) of monomethylamine, the mixture of methanol 29.87% (mass fraction) and sodium methanol 0.5% (mass fraction) (0.72% for monomethyl amine) was supplied to the reactor with 87.05 kg/h, and carbon monoxide 56.5 kg/h with 99.5% content. The reaction temperature was 90℃ and the pressure was 2.53MPa. The mixture containing 80.5% (mass fraction) of monomethylamine was taken out 140.75 kg per hour, that is, the yield of N-Methylformamide was 113.4 kg per hour, and the production capacity of 1L reactor was 1.89 kg of N-Methylformamide per hour.
2. Methyl formate method
CH3NH2+HCOOCH3 →HCONHCH3+CH3OH
The product was obtained by initial distillation and rectification with a total yield of 90%. Product content 95%~99.5% above.
3. Ethyl formate method
HCOOC2H5+CH3NH2 → HCONHCH3+C2H5OH
First, ethyl formate was added to the reaction tank, and then methylamine aqueous solution [ethyl formate and methylamine aqueous solution were mixed at 1:1.2 (mol)] under cooling. The mixture was stirred by reflux at 40℃, then placed for 3 days, and ethanol was recovered under reduced pressure. The crude product was distilled under reduced pressure to obtain the finished product
Application
Excess molar volumes and excess Gibbs energies
Excess volumes V-E measured in a dilution dilatometer and total vapor pressures at the temperature 303.15 K are reported for (N-methylformamide + water), (N-methylformamide + methanol), and (N-methylformamide + ethanol) over the entire composition range. The total vapor pressure measurements were made by a modified static method. The excess molar volumes are negative for all, the mixtures over the entire composition range; the vapor pressure measurements show only small negative deviations from ideality for these systems [1].
Vapor-liquid equilibria
In Harris’s work experimental VLE data are presented for N-methylformamide + hexane, N-methylformamide + benzene, N-methylformamide + chlorobenzene, and N-methylformamide + acetonitrile at 363.15 K. The data were measured using a computer driven static vapor-liquid equilibrium device. The data were modeled with the NRTL excess Gibbs energy (g(E)) model. Activity coefficients at infinite dilution for the low boiling compound were calculated using a differential pressure method and the NRTL equation [2].
As a potential therapeutic approach in colon-cancer
The effect of N-methylformamide, used in combination with the antineoplastic drugs adriamycin and cisplatin, on the cell survival of a colon carcinoma cell line (HT-29) was investigated. To better understand the mechanism involved in N-methylformamide-mediated chemosensitization, we evaluated the N-methylformamide effect on cell volume and surface expression of some integrins molecules (VLA2, VLA5, and VLA6) of the HT-29 cell line. Methods: The cell survival was evaluated by clonogenic assay; integrins surface expression was analyzed by means of flow cytometry; cell volumes were determined using a Coulter Channalyzer. Results: A Noncytotoxic dose of N-methylformamide (170 mM) sensitizes the HT-29 cell line to the lethal activity of both adriamycin and cisplatin. The analysis of cell volume showed that N-methylformamide exposure induces an increase in cell volume. Flow cytometric analysis of VLA2, VLA5, and VLA6 receptors showed that N-methylformamide increases the expression of the three integrins by 30 to 40 percent. Conclusion: The plasma membrane could constitute one of the N-methylformamide targets and might be involved in the differentiation and chemosensitizing effects caused by this agent. Moreover, N-methylformamide could improve colon cancer treatment when used in combination with antineoplastic drugs [3].
References
[1] Zielkiewicz J. Excess molar volumes and excess Gibbs energies in N-methylformamide+ water, or+ methanol, or+ ethanol at the temperature 303.15 K[J]. Journal of Chemical & Engineering Data, 1998, 43(4): 650-652.
[2] Harris R A, Wittig R, Gmehling J, et al. Vapor? Liquid Equilibria for Four Binary Systems at 363.15 K: N-Methylformamide+ Hexane,+ Benzene,+ Chlorobenzene, and+ Acetonitrile[J]. Journal of Chemical & Engineering Data, 2003, 48(2): 341-343.
[3] Del Bufalo D, Bucci B, D'Agnano I, et al. N-methylformamide as a potential therapeutic approach in colon cancer[J]. Diseases of the colon & rectum, 1994, 37(2): S133-S137.
Lastest Price from N-Methylformamide manufacturers
US $0.00-0.00/kg2025-11-05
- CAS:
- 123-39-7
- Min. Order:
- 1kg
- Purity:
- 98%
- Supply Ability:
- 1000kg

US $10.00/kg2025-04-21
- CAS:
- 123-39-7
- Min. Order:
- 1kg
- Purity:
- 99.5%
- Supply Ability:
- 100