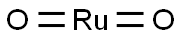Potential and proposed applications of Ruthenium Dioxide
Ruthenium oxide (RuO2), as the name denotes, is an oxide of Ruthenium. It is also referred to as ruthenium (IV) oxide, dioxoruthenium, ruthenium dioxide, ruthenia and ruthenium (IV) dioxide [1].
Potential and proposed applications of RuO2
i. Electrochemical sensors
It is reported that RuO2 is also useful as a pH sensing material; a mixture of ruthenium and titanium oxide is a precursor constituent for pH sensors. Lenz J. et al. prepared, by electrodeposition, porous microelectrodes to upgrade the pH sensing of its surface. They observed that electrodeposition of RuO2 forms a rough textured, highly active and irregularly thick surface rutile phase of RuO2. Essentially, the prepared porous RuO2 electodes showed excellent reliability. Moreover, it displayed a good thermal stability and a low electrical resistance as well as increased active surface with low Ru content and reduced effects of thermal noise [5].
Lenz J. et al. also noted that RuO2 surface has exhibited capability to oxidize nicotinamide adenine dinucleotide (NADH); a necessary step in the development of amperometric biosensors. They demonstrated that RuO2 activates the direct oxidation of NADPH at potentials as low as +200 mV, without any mediators. They also illustrated that using porous RuO2 causes notable improvement on the oxidation current that would not be attained by using a flat electrode.
ii. Capacitors
Studies have demonstrated that armophous hydrous ruthenium oxides (RuO2•xH2O, x = 0.5) exhibits a high specific capacitance of over 720 F g–1. Park S. H. et al. ascertained the pseudocapacitive properties of nano-structured anhydrous RuO2. They prepared RuO2 thin films by electrostatic spray deposition and electrochemical lithiation/delithiation. They noticed that electrochemical lithiation/delithiation of the RuO2 thin film electrode at various charge/discharge rates (C- rate) was closely linked to the specific capacitance and the capability of the nano-staructured anhydrous RuO2 thin film. They observed that the thin films prepared by electrochemical lithiation/delithiation at 2C rate showed the highest specific capacitance of 653 F g–1 at 20 Mv s-1. Furthermore, it showed deficit in specific capacitance from 653 F g–1 at 20 Mv s-1 to 559 F g–1 at 200 Mv s-1, thus showing vital advancement in the high rate capability.
Liu C. et al. also documented that among metal oxides, hydrous and amorphous RuO2 presents superior performance resulting from its high specific capacitance (1450 F g −1 of RuO2 and 1360 F g −1 of RuO2•0.5H2O over 1 V window). Their investigation involved Ni nanodendrite (ND) of diameter ≈30–100 nm directly synthesized on Ni foam and utilized as an effective support for hydrous RuO2 in symmetric supercapacitors operated at 1.6 V. They noted that highest specific capacitance of 678.57 F g −1 is achievable with energy density 60.32 Wh kg −1. They further accounted that at large current density of 100 A g −1, high energy density 19.73 Wh kg −1 can still be maintained with power density 40 kW kg −1 due to the nanostructure of the original metal.
Jian S. Y. et al. prepared a novel type of RuO2-modified multi-walled carbon nanotube (MWNT) nanocomposite electrode (RuO2/MWNT) for supercapacitors. This material was developed by depositing Ru by magnetic-sputtering in an Ar/O2 atmosphere onto MWNTs synthesized on Ta plates by chemical vapor deposition. Results showed that the capacitance of the MWNT electrodes in 1.0 M H2SO4 was significantly increased from 0.35 to 16.94 mFcm-2 by modification with RuO2. The results gave a promising path to prepare RuO2/MWNT-based double layer supercapacitors.
iii. RuO2 as an anode in a hybrid capacitor with cobalt oxide as cathode
Amorphous RuO2 is considered a promising material for a higher energy demanding power source because charge can be stored longer in the bulk of this material. It is reported that the nanocomposite of RuO2/CNTs and Co(OH)2/CNTs for ECs possess high capacitance and low resistance. Wang et al. presented this new nanocomposite hybrid capacitor with an operating voltage of 1.4 V, high energy and high power density. In their study, they emphasized that the maximum energy density and specific power density of the cell reached the value of 23.7 Wh•kg-1 and 8.1 kW•kg-1, respectively. Their hybrid capacitor delivered high power without profound loss in energy.
iv. Detection of Ranitidine
William R. et al. developed an analytical method for ranitidine quantification based on electrocatalytic oxidation of ranitidine on a glassy carbon electrode modified by electrochemical deposition of ruthenium oxide hexacyanoferrate ((RuOHCF)). They applied RuOHCF modified glassy carbon electrode as an electrocatalytical material for the oxidation of ranitidine. The modified electrode was used as an amperometric sensor of a flow injection analysis (FIA) system for the detection of ranitidine. The implemented FIA method achieved good analytical figures and displayed a decrease in the detection limit for quantification of ranitidine when compared with other analytical methods already reported in the literature. They observed that under ideal experimental conditions, the peak current response increased linearly with respect to ranitidine concentration over the range of 1.25–7.50 µmol L-1. The detection limit of the method was estimated to be 25 nmol L-1[1].
v. Electrochemical Detection of Persulfate
Research findings have demonstrated that persulfate induces or intensifies diseases such as asthma and skin reactions. Mahmoud et al. improvised a sensitive amperometric sensor’s immobilization surface for the determination of persulfate by modifying a glassy carbon (GC) electrode with a nanocomposite containing RuO2 nanoparticles and thionine (TH) and celestin blue (CB). They found out that the modified electrode showed stable and reproducible electrochemical behavior at a wide pH range (2–12) and the electrodes indicated excellent electrocatalytic activity toward persulfate reduction at a potential of +0.1 V. The proposed sensor showed detection limits of 1.46 µM for the GC/RuOx/TH modified electrode and 2.64 µM for the GC/RuOx/CB modified electrode. The sensitivities were obtained as 3 nAµM-1 at a concentration range of 10 µM to 11 mM for the GC/RuOx/TH modified electrode and 1 nAµM-1 at a concentration range of 10 µM to 6 mM for the GC/RuOx/CB modified electrodes. They also pointed out that the analytical parameters were better than those reported in the literature using other dye redox-based modified electrodes [1].
vi. Electrocatalytic Oxidation of Hypoxanthine
Hypoxanthine (Hx) plays a vital role in enhancing catabolic steps of purine nucleotides after death of animals. During the catabolic steps, the enzyme, xanthine oxidase (XOD), converts hypoxanthine (Hx) to xanthine (X) in catabolic reaction steps. A study was carried out by Zen J. M et al. to determine the performance of the Nafion/leadruthenium oxide pyrochlore modified electrode (NPME) towards various organic and biomolecules oxidation reactions, including purine and pyrimidine bases. The lead-ruthenium oxide pyrochlore, featuring advantages such as high surface area and specific redox surface group, displayed superiority in catalytic and electrospecific action to Hypoxanthine (Hx). They carried out a systematic investigation on the electrocatalytic activity of the NPME on Hx using cyclic voltammetry (CV) and the appropriate mechanistic parameters were evaluated. The studies unveiled that the NPME is catalytically active and useful for Hx oxidation and the effect was demonstrated to be very useful in its direct determination. They also confirmed that Ru(IV)/Ru(VI) redox groups of the lead-ruthenium oxide pyrochlore microparticles present in the nafion film mediated the electrocatalytic oxidation of Hx on the NPME. They highlighted that the performance of this electrode is comparable with XOD enzymatic electrodes [2].
Potential applications of RuO2 as a catalyst
Oxygen evolution reaction (OER)
Research by Exner, K. S. et al. showed that the oxygen evolution reaction (OER) can efficiently be electrocatalyzed by RuO2-based electrodes. They demonstrated that the selective substitution of Ru2f atoms of the model electrode RuO2(110) with various metals allows for fine tuning and polishing of the thermodynamically determined part of the reaction barrier to the OER [3].
Platinum-ruthenium catalyst for electrooxidation of methanol Huang, S. Y. et al. have demonstrated the application of platinum-ruthenium catalyst for electrooxidation of methanol by crystalline RuO2 which, if exploited further, would lead to generation of direct methanol fuel cell (DMFC) for commercialization. In their study, Pt–Ru/C catalyst for electrooxidation of methanol was realized by an oxidation treatment at To = 520 K. They observed that the Pt–Ru/C catalyst effect can last longer because the c-RuO2 phase is stable in the electrooxidation reaction and this was attributed to a higher activity of c-RuO2 phase than metallic Ru towards oxidization of the toxic CO [4].
Scheiba F. et al. also showed that hydrous RuO2 possess novelty as a support material for fuel cell electrocatalysts for use in direct methanol fuel cells (DMFC) applications. Their tests on membrane electrode assemblies (MEAs) showed greater performance, specifically with respect to internal resistance, when compared to Pt-Ru black. The catalyst showed very high activity in CO stripping experiments performed on a full MEA, indicating high catalyst utilization, despite the comparatively low Nafion content used in the electrode layer. They also ascertained that the RuO2 increased conductivity of protons and catalyst utilization in the MEA, while the influence on the promotion of CO and methanol oxidation is restricted [5].
Oxidation of CO to CO2
Zang L. et al. illustrated that hydrous RuO2 can successfully catalyze oxidation of CO to CO2 at room temperature. During their study, they showed that the catalyst is active during CO conversion over a whole range of CO/O2 ratios with a maximum value at a partial pressure ratio (p(CO)/p(O2))CO2 of about 1/0.56. They also observed that when CO was in excess of O2, the reaction was violent, emitting flashes of red fire at the surface of the catalyst. They found out that the activity decreased gradually at the highest CO partial pressure ratio of 1/0.16; a trend they attributed to the reduction of RuIV species to lower oxidation states by CO [5].
Oxidation of ammonia into dinitrogen
A hybrid of zeolite and RuO2 has been identified by Heylen S. et al. as an excellent catalyst for the selective catalytic oxidation of ammonia into dinitrogen in oxygen, water and carbon-dioxide containing gas stream at lower reaction temperatures of 1750C. In the course of study, they observed that over the temperature range 175–4000C, RuO2@Na-Y converted all of the NH3 in the feed. Moreover, it was highly selective for N2 at 1750C, with 99% N2 selectivity. At temperatures below 1750C, the conversion of NH3 declined rapidly to a level whereby at 1500C, only about 30% NH3 conversion was attained. While at the highest temperature of 4000C, the selectivity for N2 was about 51% thereby increasing the formation of N2O, NO, and NO2. The highest level of NOx formation (49%) was observed at 4000C. RuO2@Na-Y zeolite realized the conversion of NH3 into N2 at 1750C in the presence of water vapor and CO2. When the reaction temperature was increased, there was formation of more-oxidized reaction products including N2O, NO, and NO2 [17].
References
1.https://www.americanelements.com/ruthenium-oxide-12036-10-1
2.Hones, B. P., Løvy, F., Gerfin, T., & Grätzel, M. (2000). MOCVD of Thin Ruthenium Oxide Films : Properties and Growth Kinetics. Chemical Vapor Deposition, 6(4), 193–198
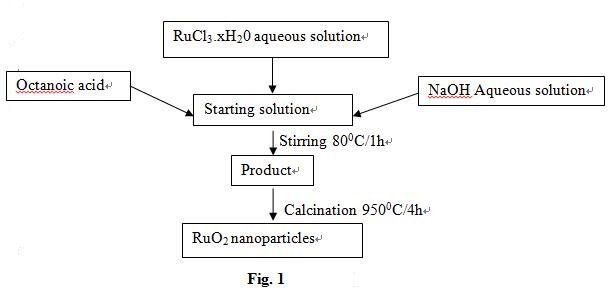
3.Khorasani-Motlagh, M., Noroozifar, M., & Yousef, M. (2011). A Simple New Method to Synthesize Nanocrystalline Ruthenium Dioxide in the Presence of Octanoic Acid As Organic Surfactant. Int. J. Nanosci. Nanotechnol, 7(4), 167–172.
4.Crihan, D., Knapp, M., Zweidinger, S., Lundgren, E., Weststrate, C. J., Andersen, J. N., Over, H. (2008). Stable Deacon Process for HCl Oxidation over RuO2 Zuschriften, 2(110), 2161–2164
5.Lenz, J., Trieu, V., Hempelmann, R., & Kuhn, A. (2011). Ordered macroporous ruthenium oxide electrodes for potentiometric and amperometric sensing applications. Electroanalysis, 23(5), 1186–1192
You may like
Related articles And Qustion
See also
Lastest Price from Ruthenium dioxide manufacturers
US $0.00-0.00/kg2025-04-04
- CAS:
- 12036-10-1
- Min. Order:
- 1kg
- Purity:
- 98%
- Supply Ability:
- 1Ton
US $0.00/kg2022-09-21
- CAS:
- 12036-10-1
- Min. Order:
- 1kg
- Purity:
- 99%
- Supply Ability:
- 1000kg