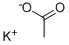Potassium Acetate: Chemical Properties, Production Methods and Medicinal Uses
Chemical Properties
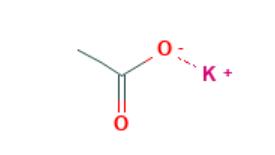
Potassium Acetate is an inorganic salt also known as potassium oxalate, diuretic salt, acetic acid, or potassium salt, with the molecular formula CH3COOK and the structure shown below. It is a deliquescent white crystalline solid with a faint acetic acid odour and a melting point of 292°C. It has a high boiling point and decomposes at high temperatures. It is insoluble in organic solvents such as ether, but soluble in alcohol, ammonia and water. The solubility in water is 100g/ml at 20 degrees Celsius.
Potassium Acetate has a pH of 7.5 to 9.0 and is considered a neutral compound. When dissolved in water, it dissociates into potassium (K+) and acetate (CH3COO-) ions. The acetate ion can act as a weak base in some reactions due to its ability to accept protons (H+), but in general, potassium acetate is not classified as an acid or a base.
Production Methods
Potassium Acetate is prepared by an acid-base neutralisation reaction in which a potassium-containing base such as potassium carbonate (K2Co3) or potassium hydroxide (KOH) is treated with acetic acid. In the neutralisation reaction, the base reacts with the acid to form a salt and water. Potassium acetate (KOH) is the salt formed when potassium hydroxide reacts with acetic acid together with water. The reaction formula is as follows:
CH3COOH+KOH→CH3COOK+H2O
Potassium acetate is also formed when small amounts of water and potassium carbonate are added to a solution of acetic acid, followed by crystallisation and evaporation. The reaction formula is:
K2Co3+2CH3COOH→2CH3COOK+H2O+CO2
Medicinal Uses
Potassium Acetate is used as a drug for the treatment of hypokalaemia in pharmaceutical applications. It is also used to treat diabetic ketoacidosis. Potassium acetate breaks down bicarbonate and helps neutralise the state of acidosis. During diabetic ketoacidosis, potassium levels in the blood are reduced. Therefore, the acetate anion present in potassium acetate can be used as it raises the levels of potassium salts to normal levels by neutralising the metabolite replacement process.
Potassium is the major cation (positive) inside animal cells, while sodium is the major cation outside animal cells. The difference in concentration of these charged particles causes a potential difference between the inside and outside of the cell, known as the membrane potential. The balance between potassium and sodium is maintained by ion pumps in the cell membrane. The cell membrane potential generated by potassium and sodium ions causes the cell to produce an action potential - a "spike" of discharge. The cell's ability to generate a discharge is critical to body functions such as nerve transmission, muscle contraction, and heart function. Potassium is also an essential mineral for regulating water balance, blood pressure and acidity.
It reduces blood pressure by eliminating salt from the body, as the potassium present in salt helps maintain blood pressure. It also helps maintain intracellular toxicity, which is necessary for smooth muscle contraction and normal kidney function.
In addition, Potassium Acetate has been used as a diuretic and urinary alkalising agent.
You may like
Related articles And Qustion
See also
Lastest Price from Potassium Acetate manufacturers

US $1200.00-1100.00/ton2025-08-11
- CAS:
- 127-08-2
- Min. Order:
- 1ton
- Purity:
- 99%
- Supply Ability:
- 1000T/M

US $10.00/KG2025-04-21
- CAS:
- 127-08-2
- Min. Order:
- 1KG
- Purity:
- 99%
- Supply Ability:
- 10 mt