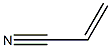Polyacrylonitrile: Characteristics and Role in Ion Exchange Resins
General Description
Polyacrylonitrile is a versatile polymer with exceptional properties, including high temperature resistance and superior tensile strength, making it ideal for applications in environmental remediation and wastewater treatment. It serves as a prominent material for producing ion exchange resins, where its insolubility allows effective ion exchange. Polyacrylonitrile can also be transformed into chelating resins to selectively extract heavy metals, demonstrating excellent adsorption capabilities. Additionally, it is utilized in developing adsorbents for radioactive waste management and can be modified to improve ion exchange properties. Overall, polyacrylonitrile plays a crucial role in advanced materials and efficient pollutant removal technologies.

Figure 1. Polyacrylonitrile
Characteristics
Polyacrylonitrile (PAN) is a crystallizing polymer that is not typical for atactic macromolecules. Its melting point is unexpectedly high (above 300 °C) and its direct determination by standard methods is complicated by the cyclization reaction that occurs at a lower temperature (approximately at 260–270 °C) than melting proceeds. In its turn, cyclization leads to the change of the backbone structure due to formation of a ladder polymer.
The first attempts to find the melting point of PAN were conducted in the 1960s–1970s by dilatometry using PAN mixtures with solvents (DMF, γ-butyrolactone) or by DTA of the AN copolymers with different comonomer (vinyl acetate) content. As a result, it was estimated that melting point of PAN lies in the range of 317–322 °C and PAN has low values of the enthalpy (ΔH = 5 kJ⋅mol−1) and entropy of melting per repeating unit (ΔS = 8 J⋅mol−1⋅K−1). The influence of the solvents on the melting point of PAN is due to the dipole–dipole interactions which appear between nitrile groups and solvent molecules. Based on these data and the X-ray diffraction results, the concept of the helical PAN conformation was formulated for the first time. It is the helical conformation that provides the formation of a long-range order during crystallization even in the absence of the configuration regularity in the chain structure. Later, the ultrafast DSC analysis at a scanning rate of 100 °C/min and higher gave rise to directly determine the melting point of PAN, the value of which coincided with the previously obtained values.1
Role in Ion Exchange Resins
Polyacrylonitrile is a prominent material used in the production of ion exchange resins, which are high-molecular-weight crosslinked polymers. These resins facilitate the exchange of ions in various solutions, making them vital in water treatment and environmental remediation applications. Polyacrylonitrile is primarily known for its insolubility in both water and organic solvents, an essential characteristic that allows it to function effectively as a solid medium for ion exchange. The development of polyacrylonitrile-based resins often employs crosslinking with other polymers, such as divinyl benzene, to enhance properties like mechanical strength and thermal stability. Different types of ion exchange resins can be created from polyacrylonitrile, including cation and anion exchangers, each designed for specific applications in ion removal, particularly of toxic metals and dyes. 2
Innovations in Chelating Resins
Polyacrylonitrile is not just utilized in standard ion exchange resins; it is also transformed into chelating resins that target heavy metals in wastewater. These chelating resins incorporate functional groups that allow for the formation of coordinate bonds with metal ions, enhancing selectivity. For example, polyacrylonitrile resins modified with amidoxime groups exhibit strong complexation capabilities, especially for metals like copper and cobalt. The complexation efficacy of polyacrylonitrile-based chelating resins has been extensively researched, with studies showing excellent adsorption performance for various heavy metals. Techniques such as redox polymerization are frequently used to create specialized polyacrylonitrile-based materials that cater to specific ion-selective applications. This innovative approach improves the overall efficiency of polyacrylonitrile resins in extracting harmful ions from industrial effluents and contaminated water bodies. 2
Applications in Environmental Remediation
The adaptability of polyacrylonitrile extends to its use in synthesizing adsorbents for radioactive waste management and heavy metal ion removal. For instance, a polyacrylonitrile-based adsorbent was created to extract cesium and cobalt ions from radioactive solutions through a composite approach with inorganic ion exchangers. This method has been shown to yield high adsorption capacities, making polyacrylonitrile a significant contributor to the field of environmental remediation. Additionally, polyacrylonitrile can undergo modification to enhance its ion exchange properties further, such as incorporating unique functional groups that improve selectivity for various metal ions. Research has demonstrated that by adjusting the synthesis conditions, such as drying temperatures, the ion exchange capacities of polyacrylonitrile composites can be optimized. Consequently, polyacrylonitrile stands as a versatile material in developing advanced technologies for ion exchange and environmental cleanup. 2
References:
[1] ELENA V CHERNIKOVA. Melt-Spinnable Polyacrylonitrile-An Alternative Carbon Fiber Precursor.[J]. Polymers, 2022, 14 23. DOI:10.3390/polym14235222.[2] ARCHANA GUPTA. A Review on Polyacrylonitrile as an Effective and Economic Constituent of Adsorbents for Wastewater Treatment.[J]. Molecules, 2022, 27 24. DOI:10.3390/molecules27248689.
You may like
Related articles And Qustion
Lastest Price from Polyacrylonitrile manufacturers

US $6.00/kg2025-04-21
- CAS:
- 25014-41-9
- Min. Order:
- 1kg
- Purity:
- 99%
- Supply Ability:
- 2000KG/Month

US $0.00-0.00/KG2025-04-15
- CAS:
- 25014-41-9
- Min. Order:
- 1KG
- Purity:
- 99%
- Supply Ability:
- 500000kg