Platinum: Major Minerals, Chemistry Properties and Major Uses
Major Minerals
Platinum is a very rare metal, occurring at a concentration of only around 0.005 ppm in the Earth's crust. Pt ( Fig1. A 3-mm cubic crystal of platinum, Pt.) is often
found as the native element and as alloys with the other Pt-group elements and Fe mostly. Most often the native Pt is
found in secondary deposits in alluvial deposits. In Ni and Cu deposits, Pt-group metals occur as sulfides (e.g., (Pt,Pd)S),
tellurides (e.g., PtBiTe), antimonides (e.g. PtSb), and arsenides (e.g., PtAs2), and as alloys with Ni or Cu. Platinum arsenide, sperrylite (PtAs2), is a major source of platinum associated with Ni ores in the Sudbury Basin deposit in Ontario,
Canada.

A total of 39 minerals are known to contain platinum in their crystal structure. The element class is represented by 12 minerals, including the native metal platinum (Pt) (Fig. 1) and 16 alloys such as isoferroplatinum (Pt3Fe) (Fig. 2), niggliite (PtSn), and tetraferroplatinum (PtFe). The sulfides class contains 22 different minerals with Pt, for example, braggite ((Pt,Pd,Ni)S) (Fig. 3), cooperite (PtS) (Fig. 4), and sperrylite (PtAs2) (Fig. 5).
FIGURE 2. Isoferroplatinum, Pt3Fe, grayish gold cubic crystal to 4 mm.

FIGURE 3. A polished slab showing the large crystal grains of ultramafic rock, Numerous metallic crystals measuring from 1 to 5 mm are braggite, (Pt,Pd,Ni)S, crystals.

FIGURE 4. Microscopic cooperite, PtS, interfaced with coarsely crystalline chromite, Fe2+Cr3+2 O4, in a matrix of pentlandite, (FexNiy)Σ9S8 (x + y = 9), and pyrrhotite, Fe7S8, about 1 cm. Associated with these minerals, though microscopic, are the platinum minerals of braggite, (Pt,Pd,Ni)S, and probable moncheite, (Pt,Pd)(Te,Bi)2.

FIGURE 5. Well-formed, complex 3 mm lustrous silvery crystal of sperrylite, PtAs2.

Chemistry Properties
Pure platinum is a lustrous, ductile, and malleable, silver-white metal (Table 7.16). Platinum is more ductile than
gold, silver, or copper, hence being the most ductile of pure metals, but it is less malleable than gold. The metal has
exceptional resistance to corrosion, is stable at high temperatures and has stable electrical properties. Platinum does oxidize, forming PtO2, at 500℃; this oxide can be readily removed thermally. It reacts vigorously with fluorine at 500℃ to form platinum tetrafluoride. It is attacked by chlorine, bromine, iodine, and sulfur. Platinum is insoluble in hydrochloric and nitric acid, but dissolves in hot aqua regia (a mixture of nitric and hydrochloric acids), to form chloroplatinic acid, H2PtCl6.
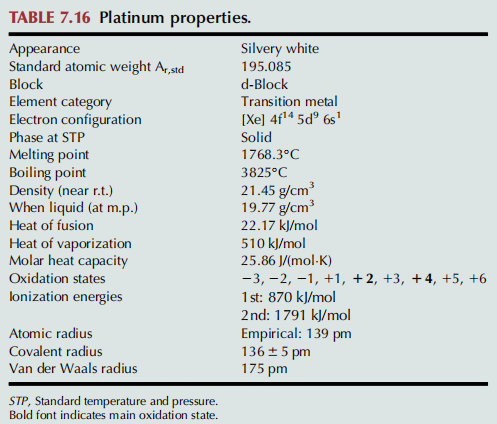
The most common oxidation states of platinum are 12 and 14. The 11 and 13 oxidation states are less common
and are often stabilized by metal bonding in bimetallic (or polymetallic) species. Tetracoordinate platinum(II) compounds tend to have 16-electron square planar geometries. While elemental platinum is generally unreactive, it dissolves in hot aqua regia to form aqueous chloroplatinic acid (H2PtCl6).
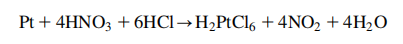
As a soft acid, platinum has a great affinity for sulfur, such as on DMSO (dimethyl sulfoxide); many DMSO complexes have been described and care should be taken in the choice of reaction solvent.
Major Uses
The most common application of Pt is as a catalyst in chemical reactions, frequently as platinum black (a fine powder of
platinum with good catalytic properties, the name of platinum black is due to its black color.) It has been used as a catalyst
since the beginning of the 19th century, when Pt powder was employed to catalyze the ignition of hydrogen. Its most significant use is in cars as a catalytic converter, which allows the complete combustion of low concentrations of unburned hydrocarbons from the exhaust into carbon dioxide (CO2) and water vapor.
In addition, Pt is employed in the petroleum industry
as a catalyst in a range of separate processes, but especially in catalytic reforming of naphthas into higher-octane gasoline
that becomes rich in aromatic compounds. PtO2, also known as Adams' catalyst, is used as a hydrogenation catalyst, specifically for vegetable oils. Adams' catalyst, also known as platinum dioxide, is usually represented as Pt(IV) oxide hydrate, PtO2· H2O. It is a catalyst for hydrogenation and hydrogenolysis in organic synthesis. This dark brown powder is commercially available. The oxide itself is not an active catalyst, but it becomes active after exposure to hydrogen whereupon it converts to platinum black, which is responsible for reactions.
Pt can be recovered from spent catalyst by conversion to ammonium chloroplatinate using
aqua regia followed by ammonia. Pt also strongly catalyzes the decomposition of hydrogen peroxide (H2O2) into water and
oxygen and it is used in fuel cells as a catalyst for the reduction of oxygen. Pt is utilized as an alloying agent for a variety of metal products, such as fine wires, noncorrosive laboratory
containers, medical instruments, dental prostheses, electrical contacts, and thermocouples. Pt-Co, an alloy of about three
parts Pt and one part Co, is employed to produce relatively strong permanent magnets.
The electrical industry employs Pt coatings for computer hard disks, thermocouples and fuel cells. The first of these is the most important, with around 80% of disks now having Pt. Modern PCs have a hard disk drive with several disks, produced from either Al or high-quality glass coated with several layers, one of which is a Co-Pt alloy with magnetic properties in which information is stored.
Related articles And Qustion
See also
Lastest Price from Platinum manufacturers

US $0.00-0.00/KG2025-11-25
- CAS:
- 7440-06-4
- Min. Order:
- 1KG
- Purity:
- 98
- Supply Ability:
- 10000KGS

US $2.00-5.00/kg2025-07-24
- CAS:
- 7440-06-4
- Min. Order:
- 1kg
- Purity:
- 99%
- Supply Ability:
- 100kg