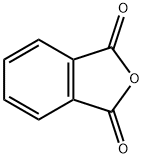
Phthalic anhydride: Pharmacodynamics, Mechanism of action, Toxicity, Applications, Preparation
Introduction
Phthalic anhydride is a white crystalline compound used primarily in the production of phthalate plasticizers, which are used to make vinyl products. It is also used as an intermediate in the production of dyes, pigments, and resins. Phthalic anhydride is highly reactive with water and alcohols, and it can cause irritation to the eyes, skin, and respiratory system if not handled properly. It is considered to be harmful if ingested or inhaled, and prolonged exposure may lead to chronic effects such as asthma or other respiratory problems[1].

Figure 1 Appearance of Phthalic anhydride
Pharmacodynamics
The phthalic anhydride can cause irritation and damage to the respiratory system if inhaled, and skin and eye irritation upon contact. Phthalic anhydride is highly reactive with water and alcohols, and can undergo hydrolysis to form phthalic acid. Phthalic acid is known to have some biological properties, including antioxidant and anti-inflammatory effects. Prolonged exposure to phthalic anhydride may lead to chronic effects such as asthma or other respiratory problems, which suggests that it may have some impact on the immune system and respiratory function. However, further research is needed to fully understand the potential pharmacodynamic effects of phthalic anhydride on human health[2].
Mechanism of action
The primary mechanism of action of phthalic anhydride is through its ability to undergo hydrolysis in the presence of water or alcohols, forming phthalic acid. Phthalic acid and its derivatives are known to have a variety of biological effects, including antioxidant and anti-inflammatory properties. Phthalic anhydride can cause irritation and damage to the respiratory system if inhaled, and skin and eye irritation upon contact. It is highly reactive, which makes it useful as an intermediate in the production of various chemicals and polymers, but also means that it can react readily with biological tissues and fluids in the body. Once absorbed into the body, phthalic anhydride can be metabolized to phthalic acid, which may then undergo further biotransformation to form other metabolites. The exact metabolic pathway of phthalic anhydride and its metabolites is not well understood and requires further research. Overall, the biological effects of phthalic anhydride appear to be primarily due to its ability to undergo hydrolysis and form phthalic acid and its derivatives, rather than any direct mechanism of action of the parent compound itself[3].
Toxicity
Phthalic anhydride is considered to be toxic, particularly if it is inhaled or ingested. Inhaling phthalic anhydride can cause irritation and damage to the respiratory system, including coughing, wheezing, and shortness of breath. It can also cause skin and eye irritation upon contact. Prolonged exposure may lead to chronic effects such as asthma or other respiratory problems. In addition to its acute toxicity, it has been classified as a potential carcinogen by some regulatory agencies. Studies have shown that chronic exposure to phthalic anhydride may increase the risk of developing certain types of cancer, particularly lung cancer. It is important to handle phthalic anhydride with care and follow appropriate safety measures, such as wearing personal protective equipment (PPE) and ensuring proper ventilation when working with this substance[4].
Applications
Phthalic anhydride is primarily used in the production of phthalate plasticizers, which are added to polyvinyl chloride (PVC) to make it more flexible and durable. These plasticizers are widely used in the manufacture of various products such as cables, flooring, roofing materials, medical devices, and other consumer goods. Phthalic anhydride is also used as an intermediate chemical in the production of a variety of other products, including dyes, pigments, resins, and polyester polymers. It is used in the production of unsaturated polyester resins, which are widely used in the construction industry for making composite materials, such as fiberglass reinforced plastics. It is also used in the production of alkyd resins, which are used as coatings for metal surfaces, wood, and other materials. In addition to these applications, phthalic anhydride is also used as a reactant in the synthesis of various organic compounds, such as pharmaceuticals and agricultural chemicals[5].
Preparation
Phthalic anhydride can be prepared by the oxidation of naphthalene or ortho-xylene. The most common method for its preparation[6] is through the catalytic vapor-phase oxidation of o-xylene, using air as the oxidant. Phthalic anhydride is an important organic chemical widely used in the production of plastics, dyes, pharmaceuticals, and other fields. The synthesis of phthalic anhydride is typically achieved by the air oxidation of ortho-xylene or naphthalene to produce phthalic acid, which is subsequently dehydrated to form phthalic anhydride. Catalysts such as cobalt, nickel, or vanadium are typically used to facilitate the reaction. Alternatively, phthalic anhydride can also be prepared by acid hydrolysis of phthalic anhydride or its derivatives.
Several variations of this process exist, including variations in temperature, pressure, catalyst composition, and reactant concentration. Other methods for preparing phthalic anhydride include the oxidation of naphthalene, although this method has largely been replaced by the more efficient o-xylene process. Overall, the production of phthalic anhydride is a highly specialized chemical process that requires careful control of reaction conditions and the use of specialized catalysts.
References
[1] Sureshkumar J, Ramaswamy D. Phthalic Anhydride: A Review. [J] Journal of Chemistry, 2017, 1-11.
[2] Lee, H. Y., Kim, J. H., Kim, J. H., et al. Phthalic acid induces inflammatory responses in mice via Toll-like receptor 4-dependent signaling pathways.[J] Korean Journal of Physiology & Pharmacology, 2016, 20(2), 157-163.
[3] Liao, C., Liu, F., Al-Mamun, M.,et al. Phthalic anhydride-induced oxidative stress and immunotoxicity in zebrafish: The role of Nrf2 and NF-κB signaling pathways.[J] Fish & Shellfish Immunology. 2020, 97, 188-196.
[4] Chen, G., Wu, Q., Zhang, J., et al. Phthalate esters in the environment: A critical review of their occurrence, biodegradation, and removal during wastewater treatment processes. [J] Science of The Total Environment, 2013,454, 202-217.
[5] Pan, J., Zhang, Q., Wang, Z., Xu, X. Simultaneous determination of phthalic acid esters in food samples by gas chromatography-mass spectrometry with a solid-phase microextraction method. [J] Journal of Chromatography A,2011, 1218(47), 8557-8563.
[6] Sun, Y., Liu, S. Process optimization for phthalic anhydride production via catalytic oxidation of o-xylene: A review.[J] Chemical Engineering Journal. 2014, 253, 579-589.
Related articles And Qustion
See also
Lastest Price from Phthalic anhydride manufacturers

US $0.00/Kg/Bag2025-04-21
- CAS:
- 85-44-9
- Min. Order:
- 1KG
- Purity:
- ≥99.5%
- Supply Ability:
- 5000mt/year

US $1.00/KG2025-03-10
- CAS:
- 85-44-9
- Min. Order:
- 1KG
- Purity:
- 99%
- Supply Ability:
- 10mt