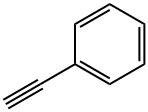
Phenylacetylene: A Hydrogen Bonding Chameleon
General description
Phenylacetylene is a natural product found in Tuber borchii and Tuber canaliculatum with data available. LOTUS - the natural products occurrence database Ethynylbenzene is a member of benzenes. All atoms of benzene are coplanar. Then, all atoms of acetylene built by carbon carbon three are also coplanar. Replace one hydrogen of benzene with carbon in acetylene. When the two are combined, all atoms are also coplanar. That is, the two planes coincide. The chemical formula is C8H6, insoluble in water and miscible in most organic solvents such as ethanol and ether. Appearance: colorless to light yellow liquid, melting point: - 44.8 ℃, boiling point: 142 ~ 144 ℃. Intermediates for organic synthesis.
Application
1.A comprehensive experiment of green polymer chemistry. Phenylacetylene was polymerized by using organic rhodium complex as the catalyst in the aqueous solution. The molecular structure and photothermal properties of phenylacetylene (PPA) were characterized by fouriter transform infrared spectroscopy (FTIR), nuclear magnetic resonance (NMR), ultraviolet-visible spectrophotometer (UV-Vis), fluorescence spectrometer and thermogravimetric analyzer[1].
2.In an attempt to address the hydrogen bonding behavior of multifunctional molecules we chose phenylacetylene (ethynyl-benzene) as a representative example. The choice of phenyl-acetylene is due to the fact that it has three hydrogen bonding sites, as illustrated in Figure 1. Two of the three sites available on phenylacetylene are hydrogen-bond acceptor sites in the form of p-electron density of the benzene ring (site A) andpelectron density of the acetylenic C≡C bond (site B). It turns out that for phenylacetylene it is not trivial to determine the propensity of the hydrogen-bond-accepting ability of these two sites within the gambit of both the Etter and Legon–Millen rules. Phenylacetylene provides aunique opportunity to test and develop new functionals based on density functional theory[2].
3.the synthesis and photophysical properties of light harvesting phenylacetylene dendrimers with unsymmetrical branching. We describe the steady state and time dependent experiments that are used to characterize energy transfer properties in the conjugated dendrimers. Finally, we describe investigations of the unsymmetrical phenylacetylene dendrimers as potential materials for applications in fluorescence-based sensors, and for non-linear optics[3].
Figure 1 Phenylacetylene: A Hydrogen Bonding Chameleon
Synthesis
It is suitable for the synthesis of Terminal Alkynes by the reaction of substituted benzaldehyde with alkali. 2-bromovinyl benzene can be obtained by reacting 2,3-dibromo-3-phenylpropionic acid with base and triethylamine in DMF, and then it can be directly reacted with potassium hydroxide to obtain the desired product Phenylacetylene[4].
Figure 2 Synthetic route of target compounds
Safety and Storage
1.Emergency treatment for leakage and cut off the fire source. Wear self-contained breathing apparatus and general fire protection clothing. Stop the leakage under the condition of ensuring safety. Spray mist can reduce evaporation. Mix and absorb with sand or other incombustible adsorbents. Then it is transported to an open place for burial, evaporation or incineration. If there is a large amount of leakage, use the embankment to receive it, and then collect, transfer, recycle or dispose it innocuously.
2.Protective measures respiratory system protection: when the concentration in the air is high, you should wear a gas mask. In case of emergency rescue or evacuation, it is recommended to wear self-contained breathing apparatus. Eye protection: wear chemical safety glasses. Body protection: wear anti-static work clothes. Hand protection: wear protective gloves. Others: smoking is strictly prohibited at the work site. Avoid long-term repeated contact.
Storage precautions: store in a cool and ventilated warehouse. Keep away from kindling and heat sources. The storage temperature should not exceed 30 ℃. The package shall be sealed and shall not come into contact with air. It shall be stored separately from oxidants, acids, halogens and alkali metals, and mixed storage shall not be allowed. It should not be stored in large quantities or for a long time. Explosion proof lighting and ventilation facilities shall be adopted. It is forbidden to use mechanical equipment and tools that are easy to produce sparks. The storage area shall be equipped with leakage emergency treatment equipment and appropriate receiving materials.
References
1.Hong Zhouyi, Zhang Jinjin, Li Yaojun, et al. "Study on coordination polymerization and photothermal properties of phenylacetylene - recommend a comprehensive experiment of green polymer chemistry", university chemistry, No. 12, 2021, pp. 86-92.
2.Peng Z., Melinger J. S. & Kleiman V., "Light Harvesting Unsymmetrical Conjugated Dendrimders as Photosynthetic Mimics," Photosynthesis Research, Vol.87, No.1(2006), pp.115-131.
3.Maity S., Guin M. & Singh P. C. et al., "Phenylacetylene: A Hydrogen Bonding Chameleon," ChemPhysChem, Vol.12, No.1(2011), pp.26-46.
4.Luo Lei, Wang Yong: "preparation of acetylene benzene with corresponding substituents from benzaldehyde with different substituents", chemical reagent, No. 05, 2021, pp. 698-704.
You may like
See also
Lastest Price from Phenylacetylene manufacturers

US $9.95/KG2020-06-02
- CAS:
- 536-74-3
- Min. Order:
- 1KG
- Purity:
- 99.50% IN STOCK
- Supply Ability:
- 500 MT per month
US $15.50/KG2020-06-02
- CAS:
- 536-74-3
- Min. Order:
- 1KG
- Purity:
- 99.50% IN STOCK
- Supply Ability:
- 500 MT per month