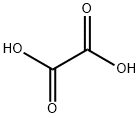
Oxalic Acid: A Comprehensive Overview
Introduction
Oxalic acid is an organic compound that is widely used in various industrial and chemical processes. Known for its strong acidic properties, it is found naturally in many plants and vegetables. In recent years, oxalic acid has garnered significant attention in the chemical community due to its diverse applications and potential health effects. This article aims to provide an in-depth overview of oxalic acid, focusing on its properties, main components, uses, and storage methods.

Figure 1 Characteristics of Oxalic acid
Properties
Oxalic acid is a white crystalline solid that is highly soluble in water. It has a melting point of 189.5°C and a boiling point of approximately 365°C. The acid is characterized by its sharp, sour taste and is known to form colorless, odorless crystals. Chemically, oxalic acid is classified as a reducing agent and can participate in various redox reactions. One of its key properties is its ability to chelate metal ions, forming stable complexes. This chelating ability makes it particularly useful in processes involving metal cleaning and extraction.
The molecular structure of oxalic acid consists of two carboxyl groups (-COOH) connected by a carbon-carbon bond. This structure allows it to donate two protons, making it a diprotic acid. The dissociation constants (pKa values) of oxalic acid are 1.25 and 4.28, indicating that it is a relatively strong acid compared to other organic acids.
Main Components
Oxalic acid itself is the primary component of interest. However, in its naturally occurring forms, it is often found in combination with other substances. For instance, in plants, oxalic acid is usually present as soluble salts such as potassium oxalate or calcium oxalate. These salts can vary in solubility, with calcium oxalate being notably less soluble in water. The presence of these salts can impact the overall behavior and reactivity of oxalic acid in different environments.
In industrial settings, oxalic acid is often produced synthetically through the oxidation of carbohydrates or the reaction of carbon monoxide with sodium hydroxide followed by acidification. The synthetic forms of oxalic acid are typically available in high purity, and suitable for various specialized applications.
Uses
Oxalic acid has a wide range of applications across different industries. One of its most common uses is in the cleaning and maintenance of metals. Due to its strong acidic and chelating properties, oxalic acid is effective in removing rust and tarnish from metal surfaces. It is also used in the textile industry for bleaching and removing stains from fabrics.
In the field of analytical chemistry, oxalic acid serves as a standard for calibrating equipment and preparing solutions for titrations. Its predictable behavior in reactions makes it a reliable reference substance.
Additionally, oxalic acid is used in the pharmaceutical industry as an intermediate in the synthesis of various drugs. It also finds applications in the production of dyes, pigments, and other chemical compounds. In agriculture, oxalic acid is utilized as a pesticide and a preservative for certain crops, helping to control pests and prolong shelf life.
Storage Methods
Proper storage of oxalic acid is crucial to ensure its stability and prevent any potential hazards. Due to its corrosive nature, oxalic acid should be stored in containers made of materials that can resist corrosion, such as high-density polyethylene (HDPE) or glass. It is essential to keep the containers tightly sealed to prevent moisture absorption, as oxalic acid is hygroscopic and can absorb water from the atmosphere, leading to clumping and degradation of the product.
Oxalic acid should be stored in a cool, dry, and well-ventilated area away from incompatible substances, such as strong bases and oxidizing agents. Direct sunlight and high temperatures should be avoided, as these conditions can accelerate the decomposition of the acid. Personal protective equipment (PPE) such as gloves, goggles, and lab coats should be worn when handling oxalic acid to prevent skin and eye contact, which can cause irritation or burns.
![Article illustration]() References
References
[1]ÇALIŞKAN, MAHMUT. "The metabolism of oxalic acid."Turkish Journal of Zoology24.1 (2000): 103-106.
[2]Williams, Hibbard E. "Oxalic acid and the hyperoxaluric syndromes."Kidney international13.5 (1978): 410-417.
You may like
Related articles And Qustion
See also
Lastest Price from Oxalic acid manufacturers

US $8.00-4.00/kg2026-02-25
- CAS:
- 144-62-7
- Min. Order:
- 1000kg
- Purity:
- 99.99%
- Supply Ability:
- 5000MT/Y

US $3.00/kg2025-04-21
- CAS:
- 144-62-7
- Min. Order:
- 1kg
- Purity:
- 0.99
- Supply Ability:
- 10000