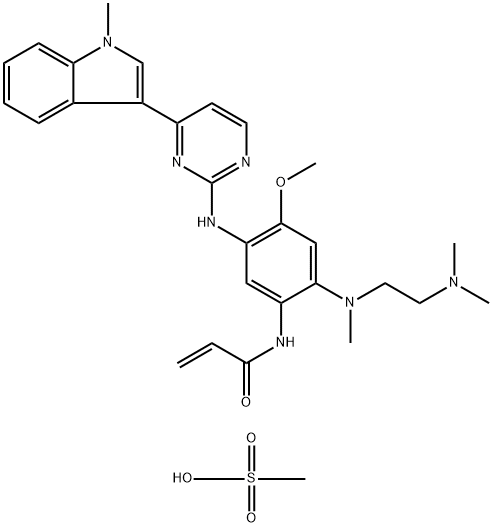
Osimertinib Mesylate: Efficacy in EGFR-Mutant NSCLC, Clinical Applications, and Resistance Mechanisms
Osimertinib mesylate is a methanesulfonate (mesylate) salt prepared from equimolar amounts of osimertinib and methanesulfonic acid. It is used for treatment of EGFR T790M mutation positive non-small cell lung cancer. It has a role as an antineoplastic agent and an epidermal growth factor receptor antagonist. It contains an osimertinib(1+). Osimertinib mesylate is approved to treat Non-small cell lung cancer that has an abnormal EGFR gene. It is used in adults: who have had surgery to remove the cancer, to help keep the cancer from coming back after surgery; with stage IIIA, stage IIIB, or stage IIIC cancer that cannot be removed by surgery and did not get worse during or after platinum-based chemoradiation therapy; as the first treatment for cancer that has spread to other parts of the body; with pemetrexed disodium and platinum-based chemotherapy as the first treatment for cancer that has spread to other parts of the body. Osimertinib mesylate is also being studied in the treatment of other types of cancer.

Osimertinib for EGFR-mutant non-small cell lung cancer
In the last 20 years the clinical management of patients with advanced non-small cell lung cancer (NSCLC) has shifted form a histology-driven to a molecularly-based approach due to the identification of actionable genetic alterations and the subsequent development of highly efficacious targeted therapies. Osimertinib mesylate is a novel pyrimidine-based irreversible, covalent third-generation EGFR-TKI and potent inhibitor of EGFR T790M mutation, the most common mechanism of acquired resistance to first-generation EGFR-TKIs. In the phase I, dose-expansion arms of AURA 1, osimertinib produced an ORR of 61% (95% CI, 52–70%), and a median PFS of 9.6 months in patients harboring the T790M mutation. As outlined by Jiang and colleagues in the consensus paper accompanying this Editorial, with the positive results of the AURA 3 and the FLAURA trials, Osimertinib mesylate has become the standard of care for treatment-naive patients with EGFR activating mutations, or EGFR T790M mutation-positive NSCLC who progress on previous EGFR-TKI treatment. However, with the increasing number of effective EGFR TKIs, and the emergence of resistance to novel agents, oncologists are faced with several questions that still need to be properly addressed. With a nearly doubled median PFS, Osimertinib mesylate is undoubtedly superior to first-generation TKIs as frontline therapy. [1]
In addition, although the OS data from the FALURA trial are still pending, the median time to second-line treatment or death was 23.5 months with Osimertinib mesylate and 13.8 months with first-line EGFR TKI, while the median time to third-line treatment was not reached and 25.9 months, respectively, suggesting an extended clinical benefit for patients starting with up-front osimertinib. Favoring this approach is also the better tolerability of osimertinib, especially because these patients are expected to remain on treatment for a longer time compared to those treated with standard EGFR TKIs. In conclusion, the question of the best treatment sequence in EGFR-mutant NSCLC has not been properly addressed yet. The phase II APPLE trial comparing upfront Osimertinib mesylate versus gefitinib followed by osimertinib at disease progression is ongoing and will help us to better understand the optimal strategy to approach patients with EGFR-mutant NSCLC. While waiting for these results and the mature OS data from the FLAURA trial, in light the impressive efficacy and the higher intracranial activity showed as frontline therapy, Osimertinib mesylate should be considered the best treatment option for all patients with newly diagnosed NSCLC harboring EGFR sensitizing mutations.
Osimertinib mesylate in the treatment of non-small-cell lung cancer
Lung cancer is the leading cause of cancer mortality, representing more than one-quarter of all cancer deaths in 2016. About 85% of lung cancers are non-small-cell lung cancer (NSCLC), comprising adenocarcinoma, squamous cell carcinoma, and large-cell lung cancer, which are generally diagnosed as locally advanced or metastatic disease. Osimertinib mesylate (TAGRISSO™, AZD9291; AstraZeneca) is a mono-anilino-pyrimidine compound that irreversibly and selectively targets EGFR TKI-sensitizing- and T790M resistance-mutant forms of EGFR, while sparing wild-type EGFR. Osimertinib binds to the EGFR kinase by targeting the cysteine-797 residue in the ATP-binding site via covalent bond formation. While WZ4002 and rociletinib share a number of common structural features, Osimertinib mesylate has a distinct, unique chemical structure. When tested across multiple other kinases, osimertinib showed minimal off-target kinase activity. At 1 μM, the compound showed inhibition >60% of a limited number of additional kinases, including ErbB2/4, ACK1, ALK, BLK, BRK, MLK1 and MNK2, thereby supporting the overall selectivity of osimertinib. Interestingly, Osimertinib mesylate did not show activity in vitro against the IGF-1R and insulin receptor, which also have a methionine gatekeeper in their kinase domains and this observation was confirmed in in vivo studies.[2]
Osimertinib has led to a paradigm shift in the management of lung cancer patients progressing to first- or second-generation TKIs, thus supporting the need in clinical practice for tumor genotyping at the time of disease progression on or after EGFR TKI therapy for biomarker evaluation of EGFR T790M. Osimertinib mesylate has also demonstrated activity in patients with CNS and leptomeningeal metastasis, which is particularly relevant, given the possibility to potentially spare these patients from radiotherapy or delay its use, including WBRT, that can ultimately compromise the cognitive function and QoL. Other novel, mutant-selective EGFR TKIs produced by different pharmaceutical companies, including EGF816, naquotinib (ASP8273), avitinib (AC0010) and PF-06747775, are in clinical development and have shown promising activity with manageable safety profile in early phase studies. Osimertinib is being tested in the first-line setting, compared with standard EGFR TKI therapy, to see whether upfront use of an irreversible inhibitor could be a more effective strategy to improve clinical outcome and delay the resistance. Despite the high efficacy of Osimertinib mesylate in T790M-mutant NSCLC, similar to other oncogene-addicted tumors, patients may ultimately progress due to acquisition of additional mechanism of resistance by tumor cells under the selective pressure of the EGFR TKI, including the C797S mutation.
Osimertinib mesylate Resistance
During the last two decades, the development and clinical application of EGFR tyrosine kinase inhibitors (EGFR-TKIs), namely the first-generation gefitinib, erlotinib, and icotinib; the second-generation afatinib and dacomitinib; and the third-generation osimertinib, have been associated with substantial responses and survival benefit in patients harboring EGFR mutations, paving the way for establishing a precision oncology approach regarding NSCLC. Osimertinib was initially approved as a second-line treatment in EGFR T790M-positive NSCLC, based on the results of the AURA trials. In 2018, osimertinib was also approved as a first-line treatment of advanced EGFR-mutant NSCLC on the basis of the results from the FLAURA trial, reporting a progression-free survival (PFS) of 18.9 months and overall survival (OS) of 38.6 months in untreated patients harboring EGFR sensitizing mutations.[3]
In this context, performing a rebiopsy upon progression to osimertinib is of crucial importance for defining further management. Whenever a tissue biopsy is feasible, it should be pursued. Otherwise, tumor genotyping with a liquid biopsy is recommended, as long as the limitations of such an approach are taken into consideration. Despite the durable responses of osimertinib, especially in the front-line setting, the majority of patients develop resistance. Research should also be focused on the standardization of liquid biopsies in order to facilitate the monitoring of molecular alterations after progression to osimertinib. The mechanisms of resistance include EGFR-dependent alterations, predominantly the acquisition of C797S mutation, which hinders the binding of the drug; and EFGR-independent mechanisms, including MET and HER2 amplifications, KRAS/BRAF/PIK3CA mutations, oncogenic fusions, and histologic transformation.
References
[1]Ricciuti B. Osimertinib for EGFR-mutant non-small cell lung cancer: place in therapy and future perspectives. J Thorac Dis. 2019 Mar;11(Suppl 3):S249-S252. doi: 10.21037/jtd.2019.01.104. PMID: 30997189; PMCID: PMC6424775.
[2]Santarpia M, Liguori A, Karachaliou N, Gonzalez-Cao M, Daffinà MG, D'Aveni A, Marabello G, Altavilla G, Rosell R. Osimertinib in the treatment of non-small-cell lung cancer: design, development and place in therapy. Lung Cancer (Auckl). 2017 Aug 18;8:109-125. doi: 10.2147/LCTT.S119644. PMID: 28860885; PMCID: PMC5571822.
[3]Gomatou G, Syrigos N, Kotteas E. Osimertinib Resistance: Molecular Mechanisms and Emerging Treatment Options. Cancers (Basel). 2023 Jan 30;15(3):841. doi: 10.3390/cancers15030841. PMID: 36765799; PMCID: PMC9913144.
Related articles And Qustion
Lastest Price from Osimertinib mesylate manufacturers
US $0.00-0.00/G2025-05-28
- CAS:
- 1421373-66-1
- Min. Order:
- 100G
- Purity:
- 99%
- Supply Ability:
- 50kgs

US $0.00/Kg/Bag2025-04-21
- CAS:
- 1421373-66-1
- Min. Order:
- 100g
- Purity:
- 99.5%min
- Supply Ability:
- 10kg