Norfloxacin: Pharmacokinetics Properties and Adverse Effects
General Description
Norfloxacin, an antibiotic used for urinary tract infections, displays peak serum concentrations 1-2 hours post oral administration. Its absorption may decrease at higher doses and be delayed by food. With renal elimination as the primary route, 30% of unchanged drug is excreted in urine. Metabolism, contributing minimally to elimination, produces metabolites with limited activity. Norfloxacin's distribution reaches various tissues, and it exhibits low protein binding. Adverse effects are generally mild, mainly gastrointestinal, and central nervous system-related, with rare instances of tendinitis. Overall, Norfloxacin is well-tolerated, showing lower incidence of adverse effects and favourable outcomes in comparative studies.

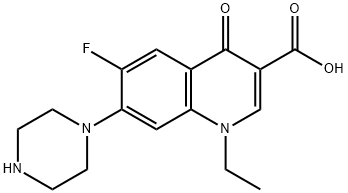
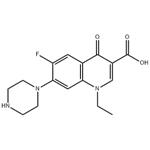
Figure 1. Norfloxacin
Pharmacokinetics Properties
Absorption and Distribution
Norfloxacin, when administered orally, exhibits peak serum concentrations typically between 1 and 2 hours post-dose. Following a single 400mg dose in healthy individuals, mean maximum serum concentrations ranged from 1.36 to 1.58 mg/L. Interestingly, with increasing doses, the peak serum concentration and overall drug exposure (AUC) decreased relative to the dose administered, indicating lower absorption rates at higher doses. Absorption of norfloxacin can be slightly delayed when taken with food. Its urinary solubility, influenced by pH, suggests a need for studying the impact of antacids on drug absorption. Approximately 28% of a single 400mg oral dose is recovered in feces over 48 hours, implying an oral bioavailability of around 70%. Regarding distribution, Norfloxacin has a significant volume of distribution, estimated to be between 25 and 35 liters based on animal studies. Concentrations in various tissues such as palatine tonsils, vaginal tissue, and gallbladder bile are comparable to or higher than serum levels. However, limited information exists on the drug's penetration into different tissues. Norfloxacin shows a protein binding level of around 14%. Research also suggests its ability to penetrate inflammatory exudates and certain tissues, while concentrations in amniotic fluid, breast milk, and umbilical serum remain low or undetectable. Further investigation is necessary to elucidate the drug's distribution dynamics comprehensively. 1
Elimination and Metabolism
Norfloxacin undergoes primarily renal elimination with approximately 30% of the unchanged drug excreted in the urine. The exact site of metabolism within the body is yet to be identified, but studies have identified six metabolites with modifications to the piperazine ring, with minimal microbiological activity. The urinary excretion of these metabolites amounts to less than 10% of the administered dose. The renal clearance of norfloxacin ranges from approximately 16 to 19.5 L/h, suggesting some degree of active tubular secretion. The administration of probenecid decreased urinary recovery, indicating the involvement of active tubular secretion mechanisms in its elimination. However, the corresponding increase in terminal serum half-life was not substantial, indicating the presence of unidentified factors influencing the drug's elimination pathway. The peak concentration of norfloxacin in urine is influenced by the volume of urine produced. Crystallization of the drug in urine has been observed at higher doses and alkaline pH values. The mean elimination half-life of norfloxacin typically falls within the range of 3.5 to 6.5 hours, with variations observed in different studies. In cases of decreased renal function, the serum half-life of norfloxacin increases, with more significant prolongation seen as creatinine clearance decreases. Dosage adjustments may be warranted in patients with impaired renal function to optimize drug therapy. Moreover, norfloxacin's metabolism plays a minor role in its elimination, as evidenced by similar serum half-lives in individuals with varying degrees of liver function impairment compared to healthy volunteers. Further research is needed to fully elucidate the drug's metabolic pathways and their implications in clinical settings. 1
Adverse Effects
Norfloxacin is a commonly used antibiotic for the treatment of urinary tract infections, and it is generally well tolerated. The overall incidence of reported adverse effects is approximately 5%, with a low incidence of abnormal laboratory values. Increasing the dosage of norfloxacin or treating neutropenic patients did not seem to significantly increase the overall incidence of drug-related side effects. The most commonly reported adverse effects of norfloxacin are disorders of the gastrointestinal tract, which occur in 2 to 4% of patients. Symptoms such as nausea and vomiting are the most frequent gastrointestinal disturbances noted. Central nervous system effects, including lightheadedness and drowsiness, are also among the more commonly reported side effects. However, these adverse effects are generally mild and only lead to withdrawal in a small percentage of patients. In some cases, tendinitis was reported in immunosuppressed individuals with poorly functioning renal transplants during long-term treatment with norfloxacin. However, symptoms resolved within 48 hours of discontinuing the antibiotic or reducing the dosage. Importantly, norfloxacin has not been associated with photosensitivity, which is a common side effect of nalidixic acid therapy. Additionally, in comparative trials, norfloxacin showed a lower incidence of superinfection with Candida and a lower development of resistant strains of Enterobacteriaceae compared to co-trimoxazole. In conclusion, while norfloxacin is generally well tolerated, it can cause gastrointestinal disturbances, central nervous system effects, and in rare cases, tendinitis. However, it is important to note that the overall incidence of adverse effects is relatively low, and norfloxacin has shown favorable outcomes in comparative trials. 2
Reference
1. Holmes B, Brogden RN, Richards DM. Norfloxacin. A review of its antibacterial activity, pharmacokinetic properties and therapeutic use. Drugs. 1985; 30(6): 482-513.
2. Rowen RC, Michel DJ, Thompson JC. Norfloxacin: clinical pharmacology and clinical use. Pharmacotherapy. 1987; 7(4): 92-110.
Related articles And Qustion
See also
Lastest Price from Norfloxacin manufacturers
US $0.00/kg2025-11-19
- CAS:
- 70458-96-7
- Min. Order:
- 1kg
- Purity:
- 98%
- Supply Ability:
- Customise

US $5.00-0.50/KG2025-06-05
- CAS:
- 70458-96-7
- Min. Order:
- 1KG
- Purity:
- 99% hplc
- Supply Ability:
- 500TONS