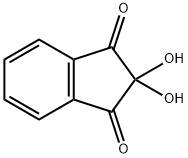
Ninhydrin hydrate Reaction and Mechanism
Ninhydrin hydrate Reaction
Ninhydrin, also known as Ninhydrin hydrate, is a chemical used to detect potential fingerprints on porous surfaces such as paper and cardboard. This compound reacts with the amino acid (eccrine) component of the fingerprint deposit to produce a dark purple product known as Ruhlmann's Violet. It can also be used to detect ammonia or primary/secondary amines. Its reaction formula is:
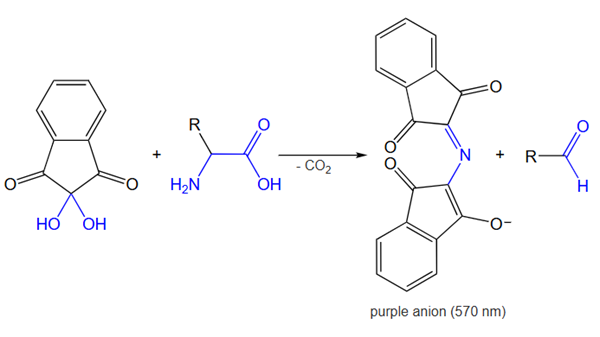
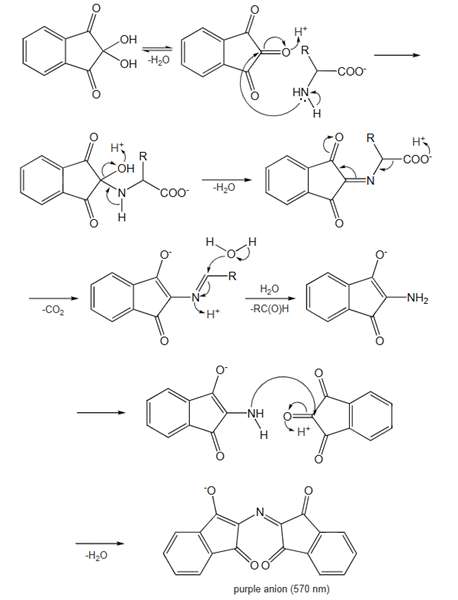
Mechanism
When a free amino acid reacts chemically with ninhydrin, which acts as an oxidizing agent, its amino group undergoes oxidative deamination, leading to the production of CO2, NH3, an aldehyde, and a reduced form of ninhydrin known as hydrindantin. Subsequently, the ammonia released reacts with another ninhydrin molecule to form the deep blue-colored diketohydrin, or Ruhemann's complex. Notably, the reaction with imino acids like proline yields a yellow complex, while asparagine results in a brown-colored complex.
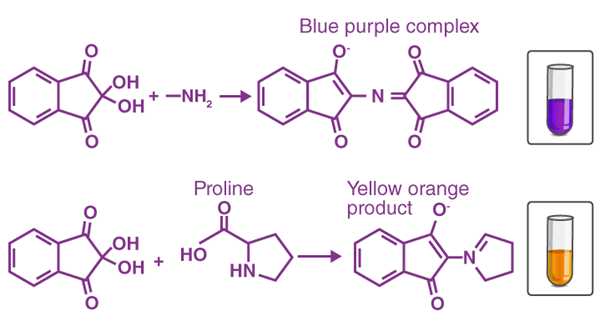
Other reactions
Ninhydrin (2,2-dihydroxy-1H-indene-1,3(2H)-dione) is an important intermediate product in the synthesis of various five-membered N,O,S-heterocyclic compounds, including pyrrole, pyrrolidine, pyrrolizidine, dihydropyrazole, imidazole, imidazolidine, di- and tetrahydrofuran, benzofuran, thiophene, oxazolidine, and thiazolidine derivatives. These heterocycles can be obtained from ninhydrin via cycloaddition, cyclocondensation, Wittig, Pictet–Spengler, Baylis–Hillman, and other reactions. Ninhydrin is frequently used in multicomponent reactions. Reactions of ninhydrin leading to the formation of six-membered N,O,S-heterocycles are also known; in particular syntheses of pyridines, piperidines, pyrimidines, piperazines, pyrazines, quinoxalines, phthalazines, pyridazines, triazines, pyrans, oxazines, and other compounds have been reported.
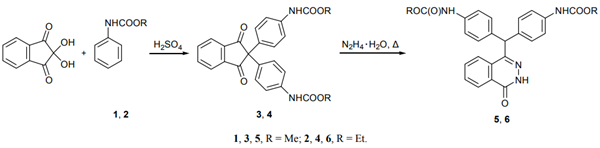
The condensation of ninhydrin with methyl and ethyl phenylcarbamates 1 and 2 in concentrated sulfuric acid at 25°C gave the corresponding para,para′-substituted bis-phenylcarbamates 3 and 4 in 94 and 97% yield with high regioselectivity. The condensation of 3 and 4 with hydrazine hydrate on heating for 15 min under reflux afforded phthalazinones 5 and 6 in 62 and 60% yield, respectively.
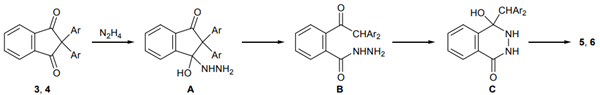
The reaction likely proceeds through nucleophilic addition of hydrazine to a ninhydrin carbonyl group, forming intermediate A. This is followed by C-C bond cleavage in A's ring, leading to intermediate B, which then cyclizes to form phthalazine 5 or 6 upon water elimination.
References:
[1] A. V. VELIKORODOV. Synthesis of New Functionally Substituted Aryl- and Hetarylcarbamates Based on Ninhydrin[J]. Russian Journal of Organic Chemistry, 2018, 54 10. DOI:10.1134/S1070428018100123.Related articles And Qustion
Lastest Price from Ninhydrin hydrate manufacturers

US $10.00/KG2025-03-10
- CAS:
- 485-47-2
- Min. Order:
- 1KG
- Purity:
- 99%
- Supply Ability:
- 10 mt
US $0.00/kg2025-03-03
- CAS:
- 485-47-2
- Min. Order:
- 1kg
- Purity:
- 99%
- Supply Ability:
- 10000KGS