Nifuroxazide: an effective therapy for acute diarrhea
Description
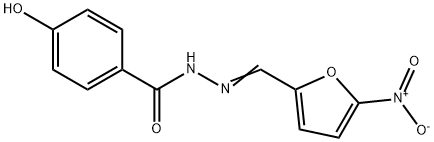
Nifuroxazide (NFX), also known as 4-hydroxy-N'-[(5-nitrofuran-2-yl) methylidene] benzo-hydrazide, is a nitrofuran derivative with antibacterial properties that have been known since 1944. Since 1966, NFX has been used for its broad-spectrum antibacterial action to treat infectious traveler diarrhea or colitis[1].

Nifuroxazide is an effective therapy for acute diarrhea and is prescribed from the onset of diarrhea without waiting for stool culture results, which can be late or negative. It is a poorly absorbed, nitrofuran derivate, anti-infective agent. The first comparative study showed that orally administered nifuroxazide had demonstrated better efficacy than probiotics in treating acute diarrhoea[2].
Biological activity
Although there is little information on the drug's activation and mechanism of action, there is a similarity between NFX's pharmacological properties (bacteriostatic and bactericidal) and those of nitrofurantoin (NFT). Nifuroxazide is an antiparasitic or amoebic drug. Nifuroxazide works by blocking the reproduction of certain substances necessary for the parasite's survival.
NFX has bactericidal activity at high concentrations, but it has bacteriostatic activity at low doses. NFX has bactericidal effects against Gram-positive bacteria such as Staphylococcus and Gram-negative bacteria such as Enterobacteriaceae: Escherichia spp., Salmonella spp., Enterobacter spp., Yersinia spp., and Klebsiella spp. On the other hand, it does not affect the bacteria Pseudomonas aeruginosa, Proteus mirabilis, and Proteus vulgaris. Because it is efficient against most intestinal infections, NFX is widely used to treat chronic and acute diarrhea caused by bacteria.
Adverse effects
The broad-spectrum intestinal anti-infectious activity of NFX makes it suitable for colitis and diarrhoea treatment. Advantageously, even at high dosages, NFX does not affect the integrity of intestinal flora. However, the drug is poorly absorbed from the digestive tract, resulting in rapid removal from the body without being metabolized. Nevertheless, NFX is contraindicated for pregnant (despite lack of evidence of its teratogenicity) and breastfeeding women as a precautionary measure[3]. Using NFX in combination with metronidazole, cephalosporins, chloramphenicol, NFT, griseofulvin, and sedatives is not recommended as interaction may cause a disulfiram-like reaction. This reaction (inhibition of hepatic aldehyde dehydrogenases (ALDH) results in the accumulation of toxic amounts of acetaldehyde in the blood) is characterized by severe nausea and vomiting, headache, and rapid heartbeat.
References
[1] Hanan S. Althagafy . “Pharmacological updates of nifuroxazide: Promising preclinical effects and the underlying molecular mechanisms.” European journal of pharmacology 951 (2023): Article 175776.
[2] Azra Husic-Selimovic. “Nifuroxazide Has Better Efficacy Than Probiotic Treatment in Adult Patients with Acute Diarrheal Syndrome.” Materia Socio-Medica 34 4 (2022): 267–271.
[3] Nonkululeko H. Zuma, David D. N’Da, Janine Aucamp. “An update on derivatisation and repurposing of clinical nitrofuran drugs.” European Journal of Pharmaceutical Sciences 140 (2019): Article 105092.
See also
Lastest Price from Nifuroxazide manufacturers

US $0.00/Kg/Drum2025-04-21
- CAS:
- 965-52-6
- Min. Order:
- 1KG
- Purity:
- 98.5%-101.5% EP
- Supply Ability:
- 1000kg

US $10.00/KG2025-04-21
- CAS:
- 965-52-6
- Min. Order:
- 1KG
- Purity:
- 99%
- Supply Ability:
- 10 mt