Nickel(II) Hydroxide: Applications in Metal Nanoparticles Formation and Foam Synthesis of its Nanoflakes
General Description
Nickel(II) Hydroxide is a metal oxide that can be used in the preparation of nickel-cadmium batteries and catalysts for chemical reactions, as well as in the preparation of nickel-silicate nanocomposites and nickel metal nanoparticles, etc. Nickel(II) Hydroxide appears as a green crystalline powder, and produces toxic gases when heated, which are carcinogenic to humans.

Figure 1. Nickel(II) Hydroxide
Synthesis of Nickel(II) Hydroxide
Nickel(II) Hydroxide is obtained by chemical deposition method using nickel(II) nitrate hexahydrate and NaOH as raw materials.
Applications
Solutions were pre-prepared with a given concentration, based on the required volume of the nano-sized product. The metallic nickel samples were obtained from the nickel hydroxides at various temperatures (200, 220, and 280 ◦C) and reduction times (15, 30, 45, 60, 90, and 120 min). A technological scheme of the nickel nanoparticles obtained is shown in Figure 1. The synthesis conditions of the samples and their designations are presented in Table 1.

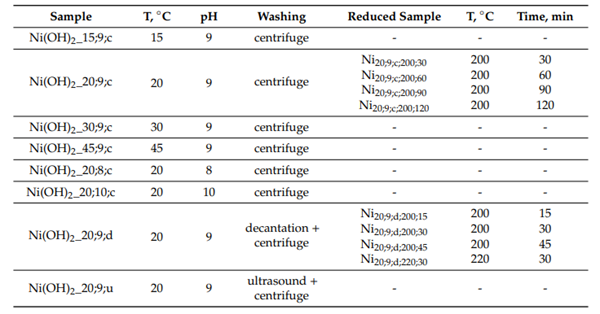
Regardless of the deposition and washing conditions, alpha modification of the nickel hydroxide with a hexagonal lattice was observed. The obtained hydroxide was an aggregate consisting of thin plates successively stacked on top of each other. The aggregate had a cylindrical shape of about a 1 nm height, with a base along the (001) plane and a height along the Z-axis. Various washing conditions lead to the formation of hydroxide with various dispersions. Decantation reduced the size of the aggregates and centrifugation reduced dispersion, evening out the effect of grinding by an ultrasonic treatment during the deposition process. Low-temperature reduction allowed us to obtain unreduced samples containing phases of both metallic nickel and nickel hydroxide.
Currently, studies have reported that amorphous mesoporous nickel/nickel(II) hydroxide (Ni/Ni(OH)2-NFs) two-dimensional nanosheets have been fabricated by chemical synthesis.Ni/Ni(OH)2-NFs nanosheets have Ni/Ni(OH)2 structure and high specific surface area (165 m2/g). The Ni/Ni(OH)2 nanosheets showed significantly enhanced electrocatalytic activity for methanol oxidation in alkaline solution compared to the parent bare-Ni(OH)2 deposited in surfactant-free solution. The high-performance electrocatalytic activity of Ni/Ni(OH)2 nanoflakes is mainly derived from efficient charge transfer due to the high specific surface area of the 2D mesoporous architecture of the nanoflakes, as well as the mass transport of methanol to Ni2+/Ni3+ active sites throughout the catalyst layer.
Nickel(II) hydroxide crystals were deposited on a substrate of layer silicates from the liquid phase by decomposing nickel(II) ammine complex solutions dispersed with expandable layer silicates; the (001) planes of the nickel(II) hydroxide aligned parallel to the basal (001) plane of the layer silicates. From these layer silicate complexes with nickel(II) hydroxide, nickel-coated layer silicate composite particulates were derived. Moreover, metal–ceramics alloys, composed of nickel containing uniformly dispersed layer silicates several tens of angstroms in thickness, were produced by sintering the metal-coated ceramic powders.
References:
[1] ELENA N SIDOROVA. Metal Nanoparticles Formation from Nickel Hydroxide.[C]//13 20. 2020. DOI:10.3390/ma13204689.[2] A. BAMUQADDAM. Foam Synthesis of Nickel/Nickel (II) Hydroxide Nanoflakes Using Double Templates of Surfactant Liquid Crystal and Hydrogen Bubbles: A High-Performance Catalyst for Methanol Electrooxidation in Alkaline Solution[J]. Nanomaterials, 2022. DOI:10.3390/nano12050879.
[3] KUNIO OHTSUKA. Epitaxial Growth of Nickel(II) Hydroxide on layer Silicate and Derived Nickel-(Layer Silicate) Nanocomposite[J]. Journal of the American Ceramic Society, 1990, 73 6: 1473-1806. DOI:10.1111/j.1151-2916.1990.tb09819.x.
See also
Lastest Price from NICKEL(II) HYDROXIDE manufacturers

US $0.00-0.00/Kg2024-12-24
- CAS:
- 12054-48-7
- Min. Order:
- 1Kg
- Purity:
- 99.9%
- Supply Ability:
- 20 tons
US $0.10/KG2024-08-05
- CAS:
- 12054-48-7
- Min. Order:
- 1KG
- Purity:
- 99.0%
- Supply Ability:
- 1000Tons