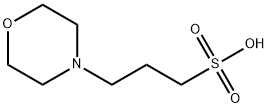
MOPS: Versatile Applications and its Production Method
General Description
MOPS (3-Morpholinopropanesulfonic acid) serves as a versatile buffering agent in biotechnology and biochemistry, ensuring pH stability during enzyme reactions and nucleic acid synthesis. Its application in medical research is crucial for maintaining optimal pH in diagnostic assays, such as ELISA kits. Additionally, MOPS aids environmental science efforts by buffering microbial cultures used in bioremediation, enhancing the degradation of pollutants. The production of MOPS involves a chemical synthesis using 1,3-bromo-chloropropane and morpholine, followed by purification through nanofiltration and ion exchange, yielding a high-quality product essential for various scientific applications.

Figure 1. MOPS
Versatile Applications
Biotechnological and Biochemical Applications
MOPS is extensively used in the field of biotechnology and biochemistry, primarily as a buffering agent in various biological and chemical processes. Its stability and minimal interference with biochemical reactions make MOPS an ideal choice for maintaining pH in environments crucial for enzyme reactions and nucleic acid research. For instance, MOPS is often used in the preparation of electrophoresis buffers, where it supports the maintenance of a stable pH, ensuring that the proteins' charge and structure remain intact during analysis. Moreover, MOPS finds applications in molecular biology, particularly in the synthesis and polymerization of nucleic acids, where precise pH control is mandatory to achieve accurate results. 1
Medical Research and Diagnostic Developments
In medical research, MOPS plays a critical role in the formulation of buffer solutions used in diagnostic assays and experimental drug formulations. Its buffering capabilities are vital for the development of immunoassay kits, where MOPS helps maintain the pH necessary for antigen-antibody reactions to occur without degradation. This characteristic is particularly significant in the context of enzyme-linked immunosorbent assays (ELISA), which rely heavily on the stability of the environment to detect the presence of antibodies or antigens in biological samples. Additionally, the use of MOPS in pharmaceutical research allows for the exploration of drug interactions under physiological conditions, providing insights into the potential efficacy and safety of new drug candidates. 1
Environmental Science and Biodegradation
MOPS is also utilized in environmental science, particularly in studies related to biodegradation and environmental pollution control. Its role as a buffer in microbial culture media enhances the growth and activity of microorganisms used in bioremediation processes. By providing a stable pH, MOPS facilitates the optimal activity of microbes that degrade toxic substances in contaminated soils and water bodies. This application is crucial in efforts to reduce the impact of industrial waste and agricultural chemicals on the environment. Moreover, the use of MOPS in such contexts supports sustainable practices by improving the efficiency of biodegradation processes, thereby contributing to the maintenance of ecological balance and reducing the reliance on chemical methods of pollution control. 1
Production Method
Production Process
The production of MOPS, involves a meticulous chemical synthesis process. Initially, 1,3-bromo-chloropropane (BCP) is combined with sodium hydroxide (NaOH) in water at a controlled temperature. Morpholine is then introduced into the mixture, which is heated and stirred for several hours to promote the reaction. The use of hydrochloric acid plays a critical role in adjusting the pH to facilitate the separation of the organic and aqueous phases, leading to the isolation of MOPS. To ensure the purity of the final product, concentrated sodium hydroxide and sodium sulfite solutions are added to reach a specific pH, followed by heating and stirring to promote further reaction and product formation. 2
Purification and Yield Enhancement
The purification of MOPS from the reaction mixture is achieved through advanced techniques such as nanofiltration and ion exchange processes. After the initial synthesis, the product solution, which contains MOPS along with various salts, undergoes nanofiltration to remove monovalent salts such as sodium chloride (NaCl) and sodium bromide (NaBr). This step is essential to reduce the osmotic pressure and concentrate MOPS effectively. The nanofiltration is conducted under specific pressure conditions and includes a diafiltration system to maintain the balance of the solution. Following nanofiltration, ion exchange processes further purify MOPS, eliminating remaining salts and yielding a final product solution with a high concentration of MOPS and minimal salt content. Overall, this detailed production and purification process ensures that MOPS is obtained in high yield and purity, bolstering its utility in various applications. 2
Reference
1. National Center for Biotechnology Information (2024). PubChem Compound Summary for CID 70807, 3[N-Morpholino]propane sulfonic acid.
2. Schaefer V; Knoll W; Schmitt A; Huettner C. Method for the production of N,N-disubstituted ω-(amino)alkylsulfonic acids. 2001; Patent Number: WO2001085678.
You may like
Lastest Price from MOPS manufacturers

US $0.00/KG2025-04-21
- CAS:
- 1132-61-2
- Min. Order:
- 1KG
- Purity:
- 99%
- Supply Ability:
- 3500kg/month

US $3.00/kg2025-04-21
- CAS:
- 1132-61-2
- Min. Order:
- 1kg
- Purity:
- 0.99
- Supply Ability:
- 1000