Minoxidil sulphate: History and Pharmacokinetics
General Description
Minoxidil sulphate, originally investigated for its potential as an anti-ulcer medication, was found to have a delayed and prolonged effect on reducing blood pressure. It was subsequently approved by the FDA in 1979 for treating hypertension under the trade name 'Loniten'. The unexpected side effect of hypertrichosis led to the development of a topical solution, which was approved in 1988 for treating male pattern baldness as 'Rogaine'. Minoxidil sulphate demonstrates different absorption characteristics depending on the route of administration. Oral minoxidil is rapidly absorbed, while absorption rates of topical solutions are relatively low. The metabolism of minoxidil sulphate occurs mainly in the liver through processes like glucuronidation, hydroxylation, and sulfation. It has minimal binding to plasma proteins and does not cross the blood-brain barrier. Excretion primarily occurs via the kidneys, both orally and topically. Understanding these pharmacokinetic aspects is crucial for optimizing treatment regimens and attaining desired therapeutic effects.

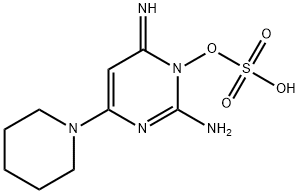
Figure 1. Minoxidil sulphate
History
Minoxidil sulphate, a derivative of N, N-Diallylmelamine (DAM), was initially investigated by Upjohn in the 1950s for its potential to treat stomach ulcers. However, instead of demonstrating anti-ulcer properties, Minoxidil sulphate was found to have a delayed and prolonged effect on reducing blood pressure. In 1968, an investigational new drug application (IND) was filed to explore minoxidil as an antihypertensive medication due to its promising results. Following successful trials with refractory hypertensive patients, oral minoxidil was approved by the FDA in 1979 under the trade name 'Loniten' for treating hypertension. Subsequently, Minoxidil sulphate's unexpected side effect of hypertrichosis, or excessive hair growth, was observed in patients taking it beyond two weeks. This led to the development of a topical Minoxidil sulphate solution, leading to the FDA approval of 2% minoxidil solution under the brand name 'Rogaine' in 1988 for treating male pattern baldness. This marked a significant milestone in the history of minoxidil sulphate, showcasing its evolution from a failed ulcer treatment to a widely-used medication for both hypertension and alopecia. 1
Pharmacokinetics
Absorption
Minoxidil sulphate, whether administered orally or topically, demonstrates different absorption characteristics. Oral minoxidil is rapidly absorbed in the gastrointestinal tract (GIT), with peak plasma concentrations achieved within an hour of administration. In contrast, the absorption rates of topical minoxidil solutions are relatively low when applied to the intact scalp. For example, the absorption rate of 2% topical minoxidil is approximately 1.4%, while that of 3% topical solution is around 1.2%. When topically applied, the serum concentration of minoxidil sulphate is usually below 5 ng/mL and can even be undetectable. A double-blind study on patients with androgenetic alopecia (AGA) found that only 7 out of 12 patients had detectable serum minoxidil levels when using a 3% minoxidil solution, with concentrations ranging from 0.4 to 7.5 ng/mL. Researchers have estimated that applying 5% topical minoxidil twice daily to the entire scalp may provide systemic exposure comparable to an oral dose of 5.4 mg. Understanding the variations in absorption between oral and topical minoxidil formulations is crucial for optimizing treatment outcomes and ensuring effective delivery of minoxidil sulphate to target tissues. 2
Distribution and metabolism
Minoxidil sulphate undergoes essential processes of distribution, metabolism, and excretion in the body. The pharmacologic action of minoxidil primarily arises from its sulfated metabolite. Upon oral administration, the majority of minoxidil is metabolized in the liver through processes such as glucuronidation, hydroxylation, and sulfation. It exhibits minimal binding to plasma proteins and does not penetrate the blood-brain barrier. In the case of topical application, follicular sulfotransferase plays a critical role in the metabolism of minoxidil sulphate. Following systemic absorption, oral minoxidil has an elimination half-life of 3 to 4 hours, with predominant excretion occurring via the kidneys within 12 to 20 hours in urine. When topically treated, approximately 95% of systemically absorbed minoxidil is eliminated within 4 days after discontinuation of the treatment. Understanding the distribution, metabolism, and excretion pathways of minoxidil sulphate is crucial for optimizing dosing regimens and ensuring effective therapeutic outcomes. 2
Reference
1. Gupta AK, Talukder M, Venkataraman M, Bamimore MA. Minoxidil: a comprehensive review. J Dermatolog Treat. 2022;33(4):1896-1906.
2. Vita Health Products Inc. Hair Regrowth Formula (Minoxidil Topical Solution USP), 2% w/v 2016.
Related articles And Qustion
Lastest Price from Minoxidil sulphate manufacturers

US $5.00-0.50/KG2025-05-08
- CAS:
- 83701-22-8
- Min. Order:
- 1KG
- Purity:
- 99% hplc
- Supply Ability:
- 500TONS

US $0.00/kg2025-04-25
- CAS:
- 83701-22-8
- Min. Order:
- 1kg
- Purity:
- 0.99
- Supply Ability:
- 1000kg