Methasterone: Functions and side effects
Introduction
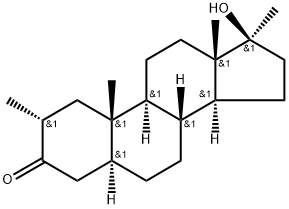
A known designer steroid is methasterone (17β-hydroxy-2α,17α-dimethyl-5α-androstan-3-one), also called 17-methyldrostanolone, which was included in the World Anti-Doping Agency (WADA) Prohibited List in 20064 and is prohibited as other steroids[1]. This drug has already been reported to cause serious liver damage in some subjects. Methasterone, C21H34O2, is a synthetic anabolic steroid known to increase weight and muscle mass.
Functions
Methasterone (superdrol and methyldrostanolone) is an orally active anabolic agent exhibiting androgenic activity. As 17α-methylation makes a valuable contribution to the enhancement of anabolic effect and considerably increases the anabolic strength of the steroid by heightening its resistance to metabolism in skeletal muscle tissue, methasterone shares many characteristic of its non-17α-alkylated counterpart drostanolone, a substance with high anabolic activity. Methasterone is also prone to be contaminated in nutritional supplements, and athletes may favor methasterone for its moderate anabolic properties and fat-burning ability. Thus, the World Anti-Doping Agency (WADA) prohibits its use in and out-of-competition. As for its damage to athletes, it is noted that the addition of a methyl group at carbon 17-α can delay methasterone eliminating from body and increase hepatic toxicity.
Metabolite
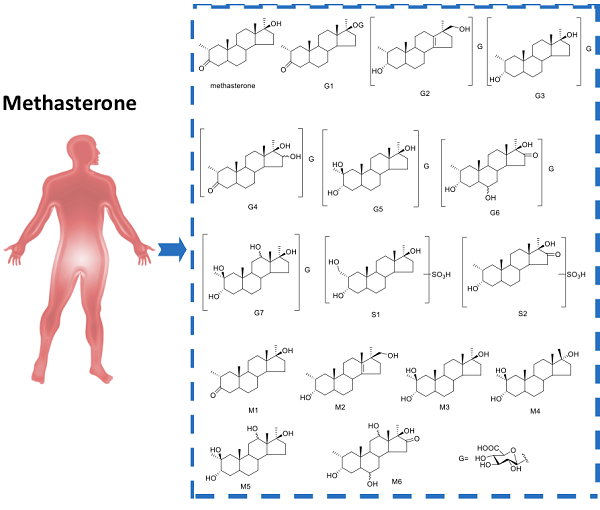
Owing to the lack of an ionizable moiety, methasterone metabolism has been investigated mainly by gas chromatography-mass spectrometry (GC-MS). Human liver microsomes and an uPA+/+-SCID chimeric mouse model were employed to produce its different metabolites, and the reduction in C3 and hydroxylation in C2, C12 (minor), C16 and C20 constitute the main metabolism pathways. Methasterone and its hydroxylated metabolites were also excreted as glucuronidated compounds, while no sulfated metabolites could be detected in phase II metabolism. Up to date, methasterone and its main metabolite (dihydromethasterone) in free and glucuronide fractions were detected in human urine by GC-MS after taking the nutritional supplement containing 10 mg methasterone and the amount of dihydromethasterone was five times greater than that of parent drug[2].
Side effects
Methasterone is hepatotoxic, and its use by athletes prompted the World Anti-Doping Agency (WADA) to impose a ban in 2006[3]. In addition to hepatotoxicity, the regular use of methasterone is associated with renal toxicity, scleral icterus, and jaundice. Biotransformation of 1 was previously reported via human hepatocytes by Gauthier et al.
References
[1] Wendell Magalhães. “Human Metabolism of The Anabolic Steroid Methasterone: Detection and Kinetic Excretion of New Phase I Urinary Metabolites and Investigation of Phase II Metabolism by GC-MS and UPLC-MS/MS.” Journal of the Brazilian Chemical Society (2019).
[2] Jianli Zhang. “New Potential Biomarker for Methasterone Misuse in Human Urine by Liquid Chromatography Quadrupole Time of Flight Mass Spectrometry.” International Journal of Molecular Sciences 17 1 (2016).
[3] L. Lootens . “Metabolic studies with promagnon, methylclostebol and methasterone in the uPA+/+-SCID chimeric mice.” Journal of Steroid Biochemistry and Molecular Biology 127 3 (2011): Pages 374-381.
See also
Lastest Price from Methasterone manufacturers

US $0.00/kg2026-03-03
- CAS:
- 3381-88-2
- Min. Order:
- 1kg
- Purity:
- 99%
- Supply Ability:
- 99999

US $10.00/box2026-01-28
- CAS:
- 3381-88-2
- Min. Order:
- 1box
- Purity:
- 99
- Supply Ability:
- in stock