Mesitylene: Applications in various fields
General Description
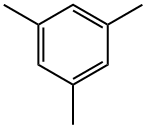
Mesitylene, also known as 1,3,5-trimethylbenzene, is a fascinating organic compound that holds great promise in various fields, particularly in environmental and sustainable technologies. With its unique chemical properties and versatility, mesitylene has emerged as a key player in addressing pressing global challenges. Mesitylene's versatility and unique properties have positioned it as a valuable compound in various environmental applications. From catalysis to energy storage, environmental remediation, and alternative fuels, mesitylene offers a range of solutions to key global challenges. Continued research and innovation in harnessing the potential of mesitylene will pave the way for a greener and more sustainable future.


Figure 1. Mesitylene
Applications
Catalysis
Mesitylene serves as an excellent precursor in catalytic reactions due to its stability and ease of functionalization. Researchers have harnessed its potential as a catalyst in organic synthesis, enabling more efficient and environmentally friendly production processes for pharmaceuticals, polymers, and fine chemicals. This application not only reduces waste but also contributes to cost-effective and sustainable manufacturing practices. The presence of mesitylene in aluminosilicate zeolite MFI two-dimensional nanosheets does not significantly impact their crystallinity, micro-/mesoporosity, or Brønsted acid site density and distribution. However, when used in benzyl alcohol alkylation reactions, mesitylene leads to a pronounced decrease in reaction rate. This reaction occurs on the external (mesoporous) surface of the nanosheets, as it cannot take place within the zeolite micropores. Transmission electron microscopy imaging reveals that the nanosheets undergo coarsening during superheated steam treatment, resulting in changes in thickness, aspect ratio, and roughness. These morphological changes on the external surface of the nanosheets are responsible for the observed variations in catalytic activity. Thus, superheated steam treatment of hierarchical zeolites provides a means to modify nanosheet morphology, regulate external surface catalytic activity, and retain the desired micro- and mesoporosity, as well as micropore reaction rates. 1
Energy storage
The electrochemical properties of mesitylene have caught the attention of scientists working on advanced energy storage systems. Mesitylene plays a crucial role in the preparation of hierarchically structured fullerene C70 cubes (HFC). It is used as a solvent in the liquid-liquid interface growth method for the formation of highly crystalline cubic shape C70 crystals (FC). The FCs are then washed with isopropanol at 25 °C to produce HFCs. The washing conditions can control the growth directions and diameters of C70 nanorods within the HFCs. These hierarchically structured HFCs exhibit excellent sensing capabilities for vapor-phase aromatic solvents. This is attributed to the easy diffusion of solvents through the mesoporous architecture of the HFCs and strong π-π interactions with the sp(2) carbon-rich pore walls. Additionally, the incorporation of mesoporous nanorods in the HFCs enhances their electrochemically active surface area, resulting in a significantly higher energy storage capacity compared to pristine C70 and fullerene C70 cubes without mesoporous nanorods. 2
Environmental Remediation
Contaminants such as volatile organic compounds (VOCs) pose significant threats to the environment and human health. Mesitylene demonstrates remarkable efficacy in adsorbing and removing VOCs from air and water. Mesitylene plays a crucial role in the synthesis of amine-enriched porous polymer gels (NUT-21-TETA). It is used as a precursor along with α, α-dichloro-p-xylene to create a low-cost and readily available monomer mixture. The two-step strategy involving nucleophilic substitution and post-synthetic amine functionalization results in the formation of NUT-21-TETA. This material exhibits a remarkable adsorption capacity for Pb2+ ions in aqueous solutions, with a maximum capacity (qm) of 1211 mg/g according to the Langmuir model. Compared to other benchmark adsorbents, NUT-21-TETA outperforms them, showcasing its potential for effective heavy metal ion removal. Additionally, NUT-21-TETA can be easily regenerated and recycled without significant loss of adsorption capacity. With its excellent performance, reusability, and cost-effectiveness, NUT-21-TETA shows great promise in practical applications for heavy metal ion trapping. 3
Reference
1. Guefrachi Y, Sharma G, Xu D, et al.
Steam-Induced Coarsening of Single-Unit-Cell MFI Zeolite Nanosheets and
Its Effect on External Surface Br?nsted Acid Catalysis. Angew Chem Int
Ed Engl, 2020, 59(24):9579-9585.
2. Bairi P, Minami K, Nakanishi W, Hill JP, Ariga K, Shrestha LK. Hierarchically Structured Fullerene C70 Cube for Sensing Volatile Aromatic Solvent Vapors. ACS Nano, 2016, 10(7):6631-6637.
3. He X, Xia J, He J, Qi K, Peng A, Liu Y. Highly Efficient Capture of Heavy Metal Ions on Amine-Functionalized Porous Polymer Gels. Gels, 2023, 9(4):297.
You may like
Related articles And Qustion
Lastest Price from Mesitylene manufacturers

US $0.00-0.00/KG2025-04-21
- CAS:
- 108-67-8
- Min. Order:
- 1KG
- Purity:
- 99%
- Supply Ability:
- 10 mt

US $75.00/KG2025-04-10
- CAS:
- 108-67-8
- Min. Order:
- 100g
- Purity:
- >99%
- Supply Ability:
- 300tons