Mesityl Oxide: Chemical Properties, Pharmacokinetics and Detection Method
General Description
Mesityl Oxide, a compound known for its stable molecular structure and low reactivity, possesses the capacity for substitution reactions under specific conditions. In pharmacokinetics, it exhibits efficient absorption across diverse routes, potentially undergoing metabolism to form glucuronide metabolites. With repeated exposure, there's a risk of accumulation, raising concerns regarding its toxicological effects. To address this, a gas chromatography method utilizing a flame ionization detector has been devised for the quantification of both mesityl oxide and diacetone alcohol within atazanavir sulfate. This method showcases remarkable specificity, sensitivity, precision, and accuracy, establishing detection limits and linearity ranges. Its efficacy in accurately determining mesityl oxide concentrations in the drug substance underscores its significance in pharmaceutical analysis and safety assessment protocols.

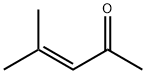
Figure 1. Mesityl oxide
Chemical Properties
Mesityl Oxide, also known as 1,3,5-trimethylbenzene oxide, possesses unique chemical characteristics that set it apart from other organic compounds. Its molecular structure, comprising three methyl groups attached to a benzene ring, with an oxygen atom bonded to one of the ring carbons, allows for specific chemical reactions. Mesityl Oxide is noted for its stability and relatively low reactivity towards common reagents. However, under certain conditions, such as the presence of strong acids or bases, Mesityl Oxide can undergo substitution reactions at the oxygenated carbon. Additionally, its aromatic nature grants it some degree of resistance to oxidation, though not as robust as unsubstituted benzene. In summary, Mesityl Oxide's chemical features stem from its distinct molecular structure, resulting in stability and specific reactivity patterns that make it a valuable compound in various chemical applications. 1
Pharmacokinetics
Mesityl oxide, a chemical compound, exhibits distinctive pharmacokinetic properties. Firstly, it is efficiently absorbed through intact skin and can also enter the body via inhalation of its vapor or ingestion. This widespread absorption mechanism indicates its potential for systemic distribution upon exposure. Research suggests that mesityl oxide undergoes reduction within the body, albeit to a significant extent. This reduction process may lead to the production of glucuronide metabolites, although direct demonstration of this metabolite is lacking. Moreover, the compound is known to interact with sulfur-containing compounds within the body, possibly sulfhydryls, forming odorous sulfur-substituted ketones. Studies involving repeated exposure of animals to non-lethal concentrations of mesityl oxide indicate that it is not rapidly eliminated. Instead, frequent exposures lead to an increase in blood concentration, eventually reaching an anesthetic level. This suggests a cumulative effect with prolonged or repeated exposure. In summary, mesityl oxide's pharmacokinetic profile highlights its efficient absorption through various routes, potential metabolism to glucuronide metabolites, interaction with sulfur compounds, and a tendency for accumulation with repeated exposures, underscoring the importance of understanding its toxicological implications in occupational and environmental settings. 2
Detection Method
A gas chromatography method employing a flame ionization detector has been devised to quantitatively determine trace levels of mesityl oxide and diacetone alcohol in atazanavir sulfate drug substance. This method utilizes a fused silica capillary column coated with a stationary phase composed of 5% diphenyl and 95% dimethyl polysiloxane (Rtx-5, 30 m x 0.53 mm x 5.0 µm), allowing for a short 20-minute runtime. Operating under programmed temperature with a split mode (1:5), the method underwent validation for specificity, sensitivity, precision, linearity, and accuracy. Detection and quantitation limits for mesityl oxide and diacetone alcohol were determined to be 5 µg/g and 10 µg/g, respectively. Linearity was established in the range of 10 µg/g to 150 µg/g with a correlation coefficient exceeding 0.999. Average recoveries in atazanavir sulfate were 102.0% to 103.7% for mesityl oxide and diacetone alcohol. The method exhibited robustness and ruggedness. Detailed experimental findings are elaborated upon in the associated research paper, affirming its efficacy for precise and reliable determination of mesityl oxide and diacetone alcohol in atazanavir sulfate drug substance. 3
Reference
1. Mesityl oxide. National Center for Biotechnology Information. 2024; PubChem Compound Summary for CID 8858.
2. MESITYL OXIDE. Hazardous Substances Data Bank. Hazardous Substances DataBank Number: 1195.
3. Raju KV, Pavan Kumar KS, Siva Krishna N, et al. Trace Level Determination of Mesityl Oxide and Diacetone Alcohol in Atazanavir Sulfate Drug Substance by a Gas Chromatography Method. Sci Pharm. 2016; 84(2): 321-331.
Related articles And Qustion
See also
Lastest Price from Mesityl oxide manufacturers

US $9.00/KG2024-10-11
- CAS:
- 141-79-7
- Min. Order:
- 1KG
- Purity:
- 99.8%
- Supply Ability:
- 100tons

US $10.00-10.00/ASSAYS2022-08-04
- CAS:
- 141-79-7
- Min. Order:
- 10ASSAYS
- Purity:
- 99%
- Supply Ability:
- 1000kg/month