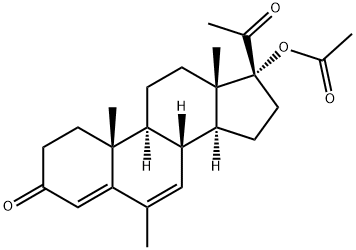
Megestrol Acetate: Anticancer Mechanisms and CYP3A4 Induction
Megestrol acetate is a type of hormone therapy. It is a synthetic form of the hormone progesterone. It disrupts the hormone balance in the body so that the body makes smaller amounts of the hormones that some cancers need to grow. It may also interact with other hormones or cause cancer cells to stop it growing. The RIA method reacts to megestrol acetate metabolites and is, therefore, non-specific and indicates higher concentrations than the GC-MF and HPLC methods. Plasma concentrations are dependent, not only on the method used, but also on intestinal and hepatic inactivation of the drug, which may be affected by factors such as intestinal tract motility, intestinal bacteria, antibiotics administered, body weight, diet, and liver function. The clinical efficacy of MEGACE Oral Suspension was assessed in two clinical trials. One was a multicenter, randomized, double-blind, placebo-controlled study comparing megestrol acetate (MA) at doses of 100 mg, 400 mg, and 800 mg per day versus placebo in AIDS patients with anorexia/cachexia and significant weight loss. Of the 270 patients entered on study, 195 met all inclusion/exclusion criteria, had at least two additional post baseline weight measurements over a 12-week period, or had one post baseline weight measurement but dropped out for therapeutic failure.

Megestrol acetate drives endometrial carcinoma cell senescence
Endometrial carcinoma (EC) is one of the most common malignant cancers among women worldwide, with increasing incidence and mortality rates. There are two predominant types of EC, Type I and Type II. Type I EC, often arising from atypical hyperplasia/endometrioid intraepithelial neoplasia, accounts for approximately 70% of all patients with endometrial cancer. Progestin therapy for the treatment of EC was proposed in 1960s, and numerous retrospective studies have been published examining the roles of hormonal therapy, including no megestrol acetate, medroxyprogesterone acetate, megestrol acetate, levonorgestrel, cyproterone acetate, hydroxyprogesterone caproate, and other unspecified/miscellaneous progestins. Senescence is a genetically regulated mechanism that involves in the normal development, and is responsible for the ending of tumor cells after chemotherapy. In endometrial cancer, downregulation of Sushi domain containing 2 (SUSD2), an endometrial mesenchymal stem cell marker, induced senescence and death of endometrial cancer cells. In human breast cancer cells, activation of PR-B by the specific ligand hydroxyprogesterone counteracted the senescence and autophagy. In the present study, we aim to investigate whether it inhibits or suppresses the development of endometrial cancer cells by activating PR-B and, to further explore the protective roles of megestrol acetate in human endometrial cancer.[1]
Megestrol is a synthetic progesterone with high efficacy. It has short-acting contraceptive role in the general use by oral or injection manner. Notably, megestrol and other progesterones are widely used in the clinical treatment of various cancers, especially malignant gynecologic carcinoma. In the treatment of advanced breast cancer, megestrol acetate provided effective palliation. The clinical safety and efficacy of megestrol application have been identified in multiple randomized, double-blind and placebo-controlled trials. However, the exact anticancer molecular mechanism involved is not completely known. PRs are the main mediators recognizing megestrol acetate and other progestagens. Decreased expressions of PR isoforms PR-A and PR-B are associated with the initiation and progression of various gynecological cancers. In breast cancer, loss of PR-B expression is observed, and hydroxyprogesterone (OHPg) can activate PR-B to further drive the autophagy in human breast cancer cells. The altered expression and functional roles of PR also have been found in endometriosis and other related diseases. In summary, the present study reveals that megestrol acetate can induce the growth arrest and senescence of endometrial cancer cells through PR-B/FOXO1/p21 axis, thus contributing to the suppression of human endometrial cancer. These findings may provide new understanding for treating human endometrial cancer.
Megestrol acetate dispersible tablets with a 5-HT3 receptor
A reasonable and effective control of chemotherapy-induced nausea and vomiting (CINV) plays an important role in the comprehensive treatment of cancer. Megestrol belongs to the 17α-hydroxyprogesterone derivative and is a highly effective synthetic progesterone. Recorded in the instructions may improve appetite and cachexia in patients with advanced tumors. In recent years, clinical practice and small sample studies have shown that megestrol combined with chemotherapy can improve CINV. This randomized controlled trial aimed to evaluate the clinical efficacy and safety of megestrol acetate combined with a 5-Hydroxytryptamine (5-HT3) receptor antagonist and dexamethasone in patients with CINV. Megestrol belongs to the 17α-hydroxyprogesterone derivative and is a highly effective synthetic progesterone. Recorded in the instructions may improve appetite and cachexia in patients with advanced tumors. In recent years, several studies have reported that megestrol acetate dispersible tablets combined with chemotherapy can improve CINV. Li et al. shows that megestrol acetate can significantly improve the appetite, body mass and Karnofsky score of patients with malignant tumor after radiotherapy, and there is no obvious adverse reaction. At present, it is considered to be an effective and safe drug for the treatment of anorexia of advanced cancer.[2]
The effect of megestrol acetate dispersible tablets combined with a 5-HT3 receptor antagonist and dexamethasone on HEC-induced CINV, especially delayed CINV, was significantly better than that of a 5-HT3 receptor antagonist combined with dexamethasone alone. The inadequacy of this study is that, this study has not designed a comparison of the efficacy of megestrol and aripipitan. Since this study only explored the effects of megestrol on CINV, future investigations comparing megestrol and arepitant will be performed. These additional clinical trials are expected to comprise a larger sample size and the influence of individual factor differences on the research results will be eliminated through the patients’ own cross-control method, so as to confirm the antiemetic effect of megestrol on acute and delayed CINV caused by HEC drugs. Moreover, megestrol acetate tablets could improve the appetite of patients, reduce myelosuppression, and improve the overall QOL of the patients, with only mild and controllable adverse reactions. Preliminary exploration of the efficacy and safety of megestrol acetate dispersible tablets in controlling HEC-induced CINV, especially delayed CINV, provides a new reference for controlling CINV in clinical practice.
Megestrol acetate as a specific inducer
Megestrol acetate is a synthetic progestogen with the same physiological effects as natural progesterone. The drug was initially developed as a contraceptive in 1963, and for over 40 years it has been used clinically for the treatment of malignancies including endometrial carcinoma, ovarian cancer, breast cancer, prostate cancer, renal cell carcinoma, hepatocellular carcinoma and malignant melanoma. It has been used for the improvement of appetite in patients receiving chemotherapy, and to relieve anorexia/cachexia syndrome in patients by using doses larger than those conventionally used to treat breast cancer. High blood sugar levels were noticed in patients taking this medication. The levels and activities of CYP3A4 were significantly induced (> 4-folds) by megestrol acetate in human hepatocytes and HepG2 cells. Megestrol treatment induced CYP3A4 through the activation of hPXR, a ligand-activated transcription factor that plays a role in drug metabolism and transport.[3]
Other tested nuclear receptors showed no response. The mechanism studies showed that megestrol activated hPXR by binding to the ligand binding domain (LBD) of hPXR and increasing the recruitment of the cofactors such as steroid receptor cofactor (SRC-1). The results suggest that megestrol acetate is a specific inducer of CYP3A4 mediated by hPXR and therefore has the potential to cause drug interactions, especially in the co-administration with drugs that are substrates of CYP3A4.
References
[1]Wang H, Shi H. Megestrol acetate drives endometrial carcinoma cell senescence via interacting with progesterone receptor B/FOXO1 axis. Exp Biol Med (Maywood). 2021 Nov;246(21):2307-2316. doi: 10.1177/15353702211026566. Epub 2021 Jul 7. PMID: 34233525; PMCID: PMC8581832.
[2]Ma Y, Zhao W, Deng W, Wei C, Bie L, Zhang C, Li N, Luo S. Megestrol acetate dispersible tablets with a 5-HT3 receptor antagonist and dexamethasone vs. 5-HT3 receptor antagonist plus dexamethasone, can better control chemotherapy-induced nausea and vomiting: a randomized controlled study. Ann Transl Med. 2022 Oct;10(20):1124. doi: 10.21037/atm-22-4809. PMID: 36388808; PMCID: PMC9652525.
[3]Chen Y, Tang Y, Nie JZ, Zhang Y, Nie D. Megestrol acetate is a specific inducer of CYP3A4 mediated by human pregnane X receptor. Cancer Chemother Pharmacol. 2021 Dec;88(6):985-996. doi: 10.1007/s00280-021-04352-9. Epub 2021 Sep 15. PMID: 34524495; PMCID: PMC8978339.
Lastest Price from Megestrol acetate manufacturers
US $0.00-0.00/KG2025-11-27
- CAS:
- 595-33-5
- Min. Order:
- 1KG
- Purity:
- 98
- Supply Ability:
- 10000KGS

US $0.00-0.00/kg2025-10-14
- CAS:
- 595-33-5
- Min. Order:
- 1kg
- Purity:
- 97.0~103.0%; USP42
- Supply Ability:
- 2000kg/month