Manganese titanate:Uses, Synthesis method, and Structure
Description
There are several ternary compounds (mixed oxides) in the Mn–Ti–O system, the most significant of which are manganese metatitanate MnTiO3 and manganese orthotitanate Mn2TiO4. In these compounds, the oxidation state of manganese is +2; that of titanium is +4. MnTiO3 is the most common form, while Mn2TiO4 is less characteristic. Under ambient conditions (1 atm, 20 °C), a stable crystalline modification of MnTiO3 is pyrophanite with the ilmenite structure.
Uses
Manganese titanate (MnTiO3) is an attractive material among all titanium-based oxides due to its great properties. For instance, the strong absorption in the visible region led to various applications such as photocatalysis and solar energy. In other words, manganese titanate's magnetic and photoelectrochemical properties are suitable for related devices. Furthermore, it has been reported to be utilized in the humidity sensors.
Synthesis method
Kharkwal et al. used oxine (8-hydroxyquinoline) as a chelate agent in order to synthesize the high surface area MnTiO3 powders at low temperatures. In this method, Ti metal powder was added to the solution of MnCl2·4H2O with an equal molar ratio under constant stirring at 70 °C. With the aid of an ammonia solution, the pH increased up to 10, and as a result, a brown-colored precipitate appeared. Afterward, the filtered precipitate was washed with water and an aqueous ammonia solution. It has been reported that at 600 °C, the formation of MnTiO3 is occurred in 6 h. The decomposition of the precursors was performed at 600 °C for 12 h, which led to a final product with black color.
Structure
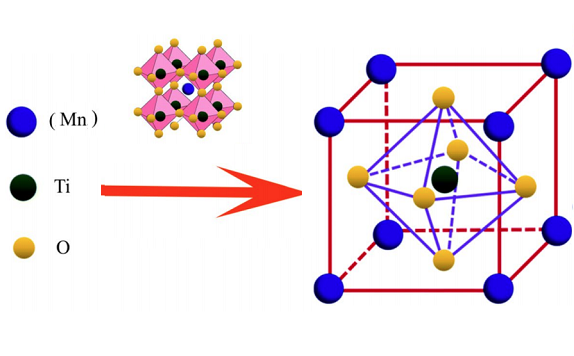
Manganese titanate has been prepared by different methods such as the chelating agent-assisted precipitation approach and sol–gel hydrothermal process. The commonly reported structure of MnTiO3, which is adopted after synthesizing, is an ilmenite structure in which Mn2+ and Ti2+ layers alternate along the c-axis of the lattice in the hexagonal setting. The calculated lattice parameters of the above products were a = 5.140 Å and c = 14.30 Å.
The transmission electron microscopy (TEM) images showed nano bars like the morphology of MnTiO3 with an average diameter of 20 nm. Strong absorption had been observed for black MnTiO3 in the visible region, which starts from 320 nm and λmax is occured at 620 nm. The charge transfer from O2− to Ti4+ is the cause of the wide absorption around 320. The absorption bands in the visible region were attributed to the 6A1g to 4A1g and 6A1g to 6T1g crystal field transition of the octahedral Mn2+ site.
References
[1] Sajad Ghaemifar. “Preparation and characterization of MnTiO3, FeTiO3, and CoTiO3 nanoparticles and investigation various applications: a review.” Journal of Materials Science: Materials in Electronics 31 9 (2020): 6511–6524.
[2] Andrey M. Abyzov, Fedor M. Shakhov, Nikolai A. Khristyuk . “Synthesis of manganese titanate and its precursors from xerogel.” Ceramics International 46 2 (2020): Pages 1990-2001.