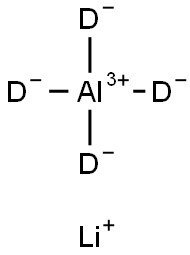Lithium aluminum deuteride: properties, applications and safety
General Description
Lithium aluminum deuteride is a stable yellow powder commonly used as a source of deuterium atoms. It has applications in the production of tritium for nuclear reactors, as a catalyst in chemical reactions, and as a potential fuel for aerospace purposes. However, it poses significant safety risks. Lithium aluminum deuteride reacts violently with water, releasing flammable gases that can ignite spontaneously. It is toxic if swallowed, causes severe skin burns and eye damage upon contact, and can be harmful when inhaled. Proper handling, storage, and ventilation are crucial to prevent accidents. Overall, Lithium aluminum deuteride is a versatile compound with important applications but requires careful management due to its hazardous nature.

Figure 1. Lithium aluminum deuteride
Properties
Lithium aluminum deuteride is a yellow powder with a density of 0.716g/cm³. This compound exhibits several distinctive properties. Firstly, it is highly stable under normal temperature and pressure conditions, making it suitable for storage and handling. However, caution should be exercised to prevent contact with water, as Lithium aluminum deuteride reacts vigorously with it. To maintain its stability, it is essential to store Lithium aluminum deuteride in a closed room at room temperature, shielded from light. Additionally, proper ventilation and drying under an inert gas atmosphere are necessary to avoid any moisture or humidity exposure. Moreover, Lithium aluminum deuteride should be kept away from acidic substances to prevent any unwanted reactions. The properties of Lithium aluminum deuteride make it a valuable compound in various applications. It is commonly used as a powerful reducing agent in organic synthesis and can facilitate the conversion of carbonyl compounds into alcohols. Additionally, LiAlD4 has been explored as a potential hydrogen storage material due to its high hydrogen content. In summary, Lithium aluminum deuteride is a stable yellow powder that reacts strongly with water. It should be stored in a closed room at room temperature, protected from light, and kept away from humid air and acids. Proper ventilation and drying under an inert gas atmosphere are crucial for its preservation. 1
Applications
Lithium aluminum deuteride is a compound that is commonly used as a source of deuterium atoms, which are heavy isotopes of hydrogen. This compound is particularly useful in the production of tritium, which is a super heavy isotope of hydrogen that is commonly used in nuclear reactors for cooling purposes. One of the primary applications of Lithium aluminum deuteride is in the production of tritium for use in nuclear reactors. Tritium is an essential component of many types of nuclear reactors, as it is used to help cool the reactor and prevent overheating. Lithium aluminum deuteride is used as a source of deuterium atoms, which can be combined with other isotopes of hydrogen to produce tritium. In addition to its use in nuclear reactors, Lithium aluminum deuteride also has a number of other potential applications. For example, it may be used as a catalyst in certain chemical reactions, or as a fuel for rockets and other aerospace applications. It may also be used as a source of deuterium in scientific research, particularly in the study of nuclear physics and other related fields. Overall, Lithium aluminum deuteride is a versatile and important compound that has a wide range of potential applications. From its use in nuclear reactors to its potential use in aerospace and scientific research, this compound is an important tool for scientists and engineers working in a variety of different fields. 2
Safety
Lithium aluminum deuteride is a compound that poses significant safety risks if mishandled. When in contact with water, it releases flammable gases that may ignite spontaneously, making it a potential fire hazard. Additionally, the compound is toxic if swallowed and can cause severe skin burns and eye damage upon contact. Furthermore, Lithium aluminum deuteride reacts violently with water, which can result in the evolution of flammable gases that may spontaneously ignite. Inhalation of the compound's fumes or vapors may cause corrosive injuries to the upper respiratory tract and lungs, making it a potential health hazard for workers who are exposed to it. In addition to its acute toxicity, Lithium aluminum deuteride is also a neurotoxin, which means that it can adversely affect the central nervous system. It is also potentially toxic to the kidneys and can cause skin burns upon contact. Overall, it is crucial to handle Lithium aluminum deuteride with care and to follow proper safety protocols when working with this compound. 3
Reference
1. PubChem. COMPOUND SUMMARY: Lithium aluminum deuteride. National Library of Medicine, 2005, PubChem CID: 11062293.
2. Ashton E, Oakley WC, Brack P, Dann SE. Evaluation of the Vapor Hydrolysis of Lithium Aluminum Hydride for Mobile Fuel Cell Applications. ACS Appl Energy Mater. 2022 Jul 25;5(7):8336-8345.
3. Hazardous Agents: Lithium aluminum deuteride. Haz-Map, CAS: 14128-54-2.
Related articles And Qustion
See also
Lastest Price from Lithium aluminum deuteride manufacturers

US $0.00-0.00/KG2025-11-24
- CAS:
- 14128-54-2
- Min. Order:
- 1KG
- Purity:
- 98
- Supply Ability:
- 10000KGS

US $10.00/KG2025-04-21
- CAS:
- 14128-54-2
- Min. Order:
- 1KG
- Purity:
- 99%
- Supply Ability:
- 10 mt