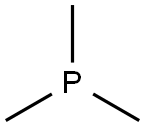
Lewis structure and polarity of trimethylphosphine
Trimethylphosphine, with the molecular formula P(CH3)3 or C3H9P, is a medium-high alkaline organophosphorus compound. With a pKa value of 8.65, it can be used as a reducing agent and neutral ligand to prepare products such as trimethylphosphine oxide, tetramethylphosphine bromide and metal complexes.
The Lewis structure of trimethylphosphine consists of a central atom phosphorus atom (P) and three methyl (CH3) groups, and each methyl (CH3) group is connected to the phosphorus atom by a single bond. The phosphorus atom (P) carries a pair of lone pairs of electrons. The specific structure is shown in the figure below:
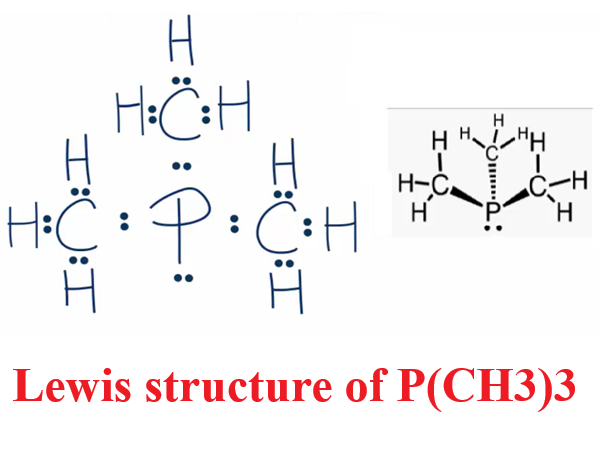
Drawing of the Lewis structure of trimethylphosphine
(1) Determine the central atom: First, it is necessary to determine the central atom in the P(CH3)3 molecule, that is, the atom with the most valence electrons in the molecule. According to the periodic table, the total number of valence electrons in the P(CH3)3 molecule is 26, that is, P(5)+[C(4)+H(3)]*3=26. And the phosphorus atom (P) has the most valence electrons, so the phosphorus atom (P) is the central atom.
(2) Determine the ligand and coordination number: The ligand is an atom or group surrounding the central atom, which forms a coordination bond with the central atom. The ligand can be an anion or a neutral molecule. The coordination number refers to the number of coordination bonds formed between the central atom and the ligand. The coordination number can usually be determined based on the number of valence electrons of the central atom and the number of ligands. In the P(CH3)3 molecule, the methyl (CH3) group is a ligand with a coordination number of 3.
(3) Determine the bonding mode: The bonding mode refers to the type of chemical bond between the central atom and the ligand. Common bonding modes include single bonds, double bonds, and triple bonds. In the P(CH3)3 molecule, a shared electron pair is formed between the phosphorus atom (P) and each ligand (CH3 group), i.e., a covalent bond (single bond). The CH3 group satisfies its eight-electron rule and achieves a stable structure. The phosphorus atom (P) retains a pair of lone pairs of electrons.
(4) Determine the stereostructure: The central phosphorus atom (P) is sp3 hybridized and contains a pair of lone pairs of electrons. Therefore, it has a pyramidal structure. The C-P-C bond angle is about 98.4°.
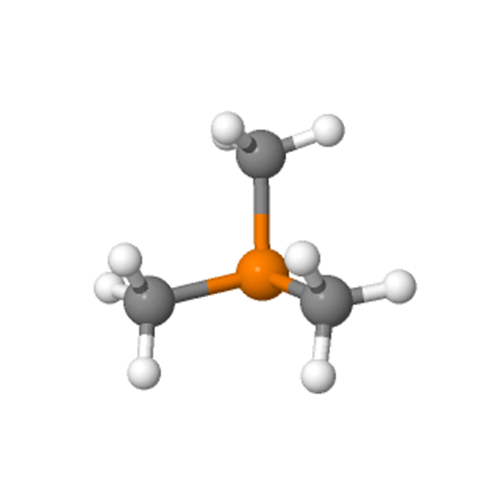
Polarity
Trimethylphosphine is a weakly polar compound with a dipole moment of 1.2 Debye and is used as a neutral ligand in coordination chemistry.
See also
Lastest Price from Trimethylphosphine manufacturers

US $0.00-0.00/KG2025-04-21
- CAS:
- 594-09-2
- Min. Order:
- 1KG
- Purity:
- 99%
- Supply Ability:
- 20 mt

US $1.00-0.00/KG2024-05-13
- CAS:
- 594-09-2
- Min. Order:
- 1KG
- Purity:
- 99%
- Supply Ability:
- 20 Tons