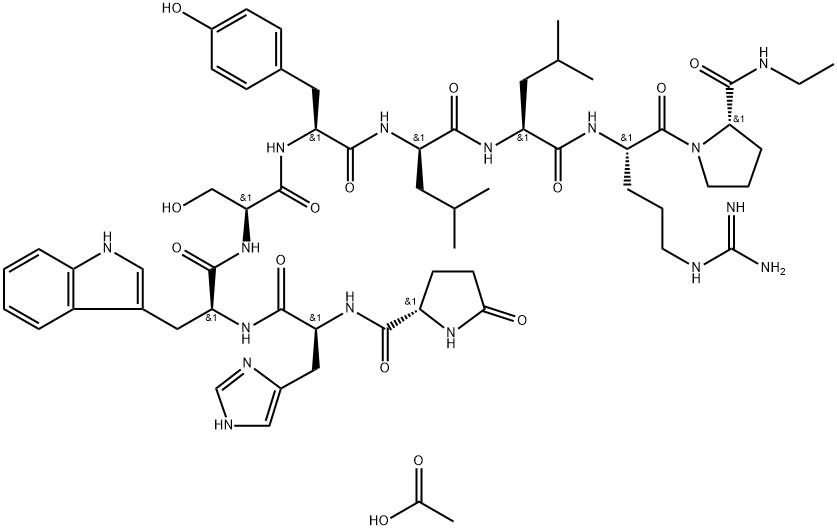
Leuprorelin Acetate: GnRH Agonist for Hormone-Driven Disorders and Long-Acting Therapies
Leuprorelin acetate injection (Eligard, Lupron Depot) is used to treat the symptoms associated with advanced prostate cancer. Leuprolide injection (Lupron Depot) is used alone or with another medication (norethindrone) to treat endometriosis. Leuprolide injection (Lupron Depot) is also used with other medication to treat anemia (a lower than normal number of red blood cells) caused by uterine fibroids (noncancerous growths in the uterus). Leuprorelin acetate belongs to a class of medications called gonadotropin-releasing hormone (GnRH) agonists. It works by decreasing the amount of certain hormones in the body.When given regularly to males, leuprolide decreases testosterone levels. Reducing the amount of testosterone in the body helps treat cancer of the prostate. When given regularly to females, Leuprorelin acetate decreases estrogen levels. Reducing the amount of estrogen in the body helps treat endometriosis. Leuprolide will also shrink tumors in the uterus, which decreases vaginal bleeding and helps prevent anemia. When given regularly to males and females who have early puberty, leuprolide slows the development of the genital area for both sexes. Leuprorelin acetate will also slow breast development in females. This medicine will delay puberty only as long as the child continues to receive it.

Clinical development of the GnRH agonist Leuprorelin acetate depot
After the isolation and chemical characterization of the gonadotropin-releasing hormone (GnRH) in 1971, drug discovery programs aimed at more potent synthetic GnRH agonists have been initiated in several pharmaceutical companies and scientific research centers, including the Salk Institute. Leuprorelin acetate (5-oxo-L-prolyl-L-histidyl-L-tryptophyl- L-seryl-L-tyrosyl-D-leucyl-L-leucyl-L-arginyl-N-ethyl-L-prolinamide acetate) is a synthetic peptide analog of GnRH with increased potency and longer half-life compared with GnRH. Subsequently, several long-acting delivery formulations using microsphere delivery system have been developed by Abbott Laboratories and Takeda. The depot formulations of Leuprorelin acetate were evaluated clinically by TAP (Takeda-Abbott Partnership) Pharmaceuticals in the United States. Based on these studies, Lupron Depot(R) has been approved in the United States by the Food and Drug Administration (FDA) and globally (under different tradenames) for different suppressive therapies in men and women, including the palliative treatment of advanced prostate cancer, management of endometriosis alone and in combination with the progestin norethindrone acetate. There is a plethora of studies with generic formulations of leuprorelin acetate, as well as indications that have not been formally approved. These studies are not included in this review.[1]
To evaluate the efficacy and safety of Leuprorelin acetate depot, alone and in combination with 3 hormonal add-back regimens, a 12-month study (n = 201) was conducted in women with endometriosis-associated pelvic pain. There were no statistically significant differences between treatment groups. The study concluded that the use of Leuprorelin acetate depot in combination with NETA 5 mg alone or with NETA and CEE 0.625 mg provides effective suppression of pelvic pain associated with endometriosis while protecting against bone loss. Leuprorelin acetate depot plus iron compared with iron alone was studied as a preoperative treatment (hysterectomy, myomectomy, endometrial ablation) in women with anemia due to prolonged or excessive bleeding associated with uterine fibroids. Leuprolide acetate was the first GnRH agonist that entered clinical development as daily subcutaneous injections in men with advanced prostate cancer and was approved by the FDA in 1985. Successively, several long-acting depot formulations of Leuprorelin acetate (ranging from 1-month to 6-month, depending on indication) have been developed for various indications in men, women, and children, including palliative treatment of prostate cancer, management of endometriosis, preoperative treatment of women with anemia and fibroids, and CPP.
A randomized controlled study evaluating safety and efficacy of leuprorelin acetate
Luteinizing hormone-releasing hormone (LH-RH) agonists provide effective adjuvant treatment for premenopausal women with endocrine-responsive breast cancer. Here, we investigated appropriate treatment durations of an LH-RH agonist, leuprorelin. Leuprorelin acetate (leuprorelin), an LH-RH agonist, is available as depot formulations for subcutaneous administration every 1- or 3-months. It is used worldwide for the treatment of hormone-responsive cancers, such as prostate cancer and premenopausal breast cancer, as well as estrogen-dependent conditions such as endometriosis and uterine fibroids. In premenopausal patients with ER-positive, node-positive breast cancer, Leuprorelin acetate administered every-3-months depot showed a non-inferior effect to chemotherapy with cyclophosphamide, methotrexate, and fluorouracil (CMF), and was well tolerated. To investigate the appropriate treatment duration for Leuprorelin acetate, we conducted an open-label, randomized controlled pilot study evaluating the safety and efficacy of adjuvant therapy with leuprorelin administered every-3-months for 2 years versus 3 or more up to 5 years in combination with tamoxifen given daily for 5 years in premenopausal women with endocrine-responsive breast cancer.[2]
Our study suggests that adjuvant Leuprorelin acetate treatment for 3 or more up to 5 years with tamoxifen for 5 years resulted in a little higher DFS rate at week 240 compared with 2 years of leuprorelin treatment with tamoxifen, in particular, during the third through fifth year study period; nevertheless, there were no significant differences between the 2 groups. The increase in the treatment duration of leuprorelin led to a decrease in the BMD, but there were neither increases in the severity of AEs nor occurrences of any new types of AEs. The safety profile of the 3 or more years of Leuprorelin acetate treatment is comparable to that of the 2 years of its treatment. No new safety signal was identified for long-term treatment with Leuprorelin acetate. These findings thus demonstrated that adjuvant leuprorelin treatment for 3 or more up to 5 years concomitant with tamoxifen for 5 years was safe and well tolerated. In addition to longer follow-up observation, further clinical trials with larger patient populations will be needed to evaluate the utility of the long-term postoperative adjuvant endocrine therapy of leuprorelin plus tamoxifen, and to determine the optimal duration of Leuprorelin acetate treatment for premenopausal women with endocrine-responsive breast cancer.
Efficacy and safety of leuprorelin acetate 6-month depot
Leuprorelin acetate is a LH–RH agonist that is commonly used worldwide. Leuprorelin acetate was initially daily administered to patients, but the development of new formulation technology enabled 1-month (USA: 7.5 mg; EU and Japan: 3.75 mg), 3-month (USA: 22.5 mg; EU and Japan: 11.25 mg) and 6-month (USA: 45 mg; EU: 30 mg) depot formulations for prostate cancer, easing the burden on patients and physicians by reducing the number of injections and improving the quality of life of patients. Subjects were excluded from the study if they met any of the following criteria: active multiple primary cancers; previous use of chemotherapy for prostate cancer; use or previous use of the marketed leuprorelin acetate as an adjuvant therapy; use or previous use of the marketed TAP-144-SR (3M) for intermittent androgen deprivation therapy. The administration period of marketed leuprorelin acetate before the start of the study drug was similar between the two treatment groups. No major differences were also observed in other subject demographics between the two treatment groups.[3]
The primary endpoint of this study was to verify the non-inferiority of TAP-144-SR (6M) compared with Leuprorelin acetate (3M) for the suppressive effect on serum testosterone. Since the lower CI of the between-group difference in the suppression rate was more than −10% of the pre-determined allowable limit value, the non-inferiority of TAP-144-SR (6M) to Leuprorelin acetate (3M) was confirmed for the suppressive effect on the serum testosterone level. The results of disease progression, as assessed by the PSA concentrations and anti-tumor effects in both treatment groups, were similar. Therefore, there were no noteworthy differences in the overall efficacy.
References
[1]Chwalisz K. Clinical development of the GnRH agonist leuprolide acetate depot. F S Rep. 2022 Nov 21;4(2 Suppl):33-39. doi: 10.1016/j.xfre.2022.11.011. PMID: 37223757; PMCID: PMC10201295.
[2]Shiba E, Yamashita H, Kurebayashi J, Noguchi S, Iwase H, Ohashi Y, Sasai K, Fujimoto T. A randomized controlled study evaluating safety and efficacy of leuprorelin acetate every-3-months depot for 2 versus 3 or more years with tamoxifen for 5 years as adjuvant treatment in premenopausal patients with endocrine-responsive breast cancer. Breast Cancer. 2016 May;23(3):499-509. doi: 10.1007/s12282-015-0593-z. Epub 2015 Feb 6. PMID: 25655898; PMCID: PMC4839052.
[3]Suzuki K, Namiki M, Fujimoto T, Takabayashi N, Kudou K, Akaza H. Efficacy and safety of leuprorelin acetate 6-month depot in prostate cancer patients: a Phase III, randomized, open-label, parallel-group, comparative study in Japan. Jpn J Clin Oncol. 2015 Dec;45(12):1168-74. doi: 10.1093/jjco/hyv149. Epub 2015 Oct 20. PMID: 26486824; PMCID: PMC4653047.
See also
Lastest Price from Leuprorelin acetate manufacturers
US $0.00-0.00/kg2025-08-22
- CAS:
- 74381-53-6
- Min. Order:
- 1kg
- Purity:
- 99%
- Supply Ability:
- 1
US $0.00-0.00/KG2025-05-09
- CAS:
- 74381-53-6
- Min. Order:
- 1KG
- Purity:
- 98.0%
- Supply Ability:
- 10000KGS